Targeting Sirt-1 controls GVHD by inhibiting T-cell allo-response and promoting Treg stability in mice
- PMID: 30514750
- PMCID: PMC6337874
- DOI: 10.1182/blood-2018-07-863233
Targeting Sirt-1 controls GVHD by inhibiting T-cell allo-response and promoting Treg stability in mice
Abstract
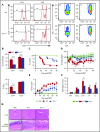
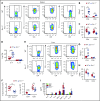
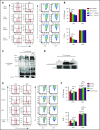
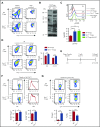
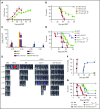
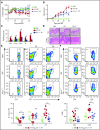
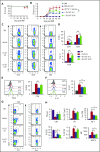
Graft-versus-host disease (GVHD) remains one of the major complications after allogeneic bone marrow transplantation (allo-BMT). Sirtuin-1 (Sirt-1) plays a crucial role in various biological processes including cellular senescence, metabolism, and inflammatory responses. Sirt-1 deacetylation regulates different transcription factors that are important for modulating immune responses. In the current study, we addressed the role of Sirt-1 in GVHD induction by employing Sirt-1 conditional knockout mice as well as a pharmacological Sirt-1 inhibitor. Using major histocompatibility complex (MHC)-mismatched and MHC-matched murine BMT models, we found that Sirt-1-/- T cells had a reduced ability to induce acute GVHD (aGVHD) via enhanced p53 acetylation. Sirt-1-deficient T cells also promoted induced regulatory T cell (iTreg) differentiation and inhibited interferon-γ production after allo-BMT. Sirt-1 deletion in iTregs increased Foxp3 stability and restrained iTreg conversion into pathogenic T cells. Furthermore, we found that administration with a Sirt-1 inhibitor, Ex-527, significantly improved recipient survival and clinical scores, with no signs of tumor relapse. These results indicate that Sirt-1 inhibition can attenuate GVHD while preserving the graft-versus-leukemia effect. Consistently, Sirt-1-deficient T cells also displayed a remarkably reduced ability to induce chronic GVHD (cGVHD). Mechanistic studies revealed that Sirt-1 deficiency in T cells enhanced splenic B-cell reconstitution and reduced follicular T helper cell development. Sirt-1 deficiency in T cells modulated donor B-cell responses reducing both B-cell activation and plasma cell differentiation. In addition, therapeutic Sirt-1 inhibition could both prevent cGVHD and reduce established cGVHD. In conclusion, Sirt-1 is a promising therapeutic target for the control of aGVHD and cGVHD pathogenesis and possesses high potential for clinical application.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

