MGMT Promoter Methylation Cutoff with Safety Margin for Selecting Glioblastoma Patients into Trials Omitting Temozolomide: A Pooled Analysis of Four Clinical Trials
- PMID: 30514777
- PMCID: PMC8127866
- DOI: 10.1158/1078-0432.CCR-18-3181
MGMT Promoter Methylation Cutoff with Safety Margin for Selecting Glioblastoma Patients into Trials Omitting Temozolomide: A Pooled Analysis of Four Clinical Trials
Abstract
Purpose: The methylation status of the O6-methylguanine DNA methyltransferase (MGMT) gene promoter is predictive for benefit from temozolomide in glioblastoma (GBM). A clinically optimized cutoff was sought allowing patient selection for therapy without temozolomide, while avoiding to withhold it from patients who may potentially benefit.Experimental Design: Quantitative MGMT methylation-specific PCR data were obtained for newly diagnosed patients with GBM screened or treated with standard radiotherapy and temozolomide in four randomized trials. The pooled dataset was randomly split into a training and test dataset. The unsupervised cutoff was obtained at a 50% probability to be (un)methylated. ROC analysis identified an optimal cutoff supervised by overall survival (OS).
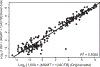
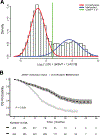
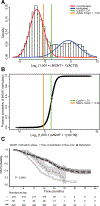
Results: For 4,041 patients valid MGMT results were obtained, whereof 1,725 were randomized. The unsupervised cutoff in the training dataset was 1.27 (log2[1,000 × (MGMT+1)/ACTB]), separating unmethylated and methylated patients. The optimal supervised cutoff for unmethylated patients was -0.28 (AUC = 0.61), classifying "truly unmethylated" (≤-0.28) and "gray zone" patients (>-0.28, ≤1.27), the latter comprising approximately 10% of cases. In contrast, for patients with MGMT methylation (>1.27) more methylation was not related to better outcome. Both methylated and gray zone patients performed significantly better for OS than truly unmethylated patients [HR = 0.35, 95% confidence interval (CI), 0.27-0.45, P < 0.0001; HR = 0.58, 95% CI, 0.43-0.78, P < 0.001], validated in the test dataset. The MGMT assay was highly reproducible upon retesting of 218 paired samples (R 2 = 0.94).
Conclusions: Low MGMT methylation (gray zone) may confer some sensitivity to temozolomide treatment, hence the lower safety margin should be considered for selecting patients with unmethylated GBM into trials omitting temozolomide.
©2018 American Association for Cancer Research.
Figures

References
-
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005;352:997–1003. - PubMed
-
- Malmstrom A, Gronberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol 2012;13:916–26. - PubMed
-
- Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol 2012;13:707–15. - PubMed
-
- Perry JR, Laperriere N, O’Callaghan CJ, Brandes AA, Menten J, Phillips C, et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med 2017;376:1027–37. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

