A unique ferredoxin acts as a player in the low-iron response of photosynthetic organisms
- PMID: 30514818
- PMCID: PMC6304933
- DOI: 10.1073/pnas.1810379115
A unique ferredoxin acts as a player in the low-iron response of photosynthetic organisms
Abstract
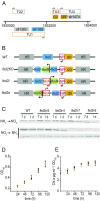
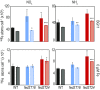
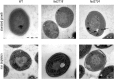
Iron chronically limits aquatic photosynthesis, especially in marine environments, and the correct perception and maintenance of iron homeostasis in photosynthetic bacteria, including cyanobacteria, is therefore of global significance. Multiple adaptive mechanisms, responsive promoters, and posttranscriptional regulators have been identified, which allow cyanobacteria to respond to changing iron concentrations. However, many factors remain unclear, in particular, how iron status is perceived within the cell. Here we describe a cyanobacterial ferredoxin (Fed2), with a unique C-terminal extension, that acts as a player in iron perception. Fed2 homologs are highly conserved in photosynthetic organisms from cyanobacteria to higher plants, and, although they belong to the plant type ferredoxin family of [2Fe-2S] photosynthetic electron carriers, they are not involved in photosynthetic electron transport. As deletion of fed2 appears lethal, we developed a C-terminal truncation system to attenuate protein function. Disturbed Fed2 function resulted in decreased chlorophyll accumulation, and this was exaggerated in iron-depleted medium, where different truncations led to either exaggerated or weaker responses to low iron. Despite this, iron concentrations remained the same, or were elevated in all truncation mutants. Further analysis established that, when Fed2 function was perturbed, the classical iron limitation marker IsiA failed to accumulate at transcript and protein levels. By contrast, abundance of IsiB, which shares an operon with isiA, was unaffected by loss of Fed2 function, pinpointing the site of Fed2 action in iron perception to the level of posttranscriptional regulation.
Keywords: FdC2; cyanobacteria; fed2; ferredoxin; iron.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Lyons TW, Reinhard CT, Planavsky NJ. The rise of oxygen in Earth’s early ocean and atmosphere. Nature. 2014;506:307–315. - PubMed
-
- Norman L, Cabanesa DJ, Blanco-Ameijeiras S, Moisset SA, Hassler CS. Iron biogeochemistry in aquatic systems: From source to bioavailability. Chimia (Aarau) 2014;68:764–771. - PubMed
-
- Margulis L. Symbiotic theory of the origin of eukaryotic organelles; criteria for proof. Symp Soc Exp Biol, 1975:21–38. - PubMed
-
- Falkowski PG, Barber RT, Smetacek V. Biogeochemical controls and feedbacks on ocean primary production. Science. 1998;281:200–207. - PubMed
-
- Coale KH, et al. A massive phytoplankton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial Pacific Ocean. Nature. 1996;383:495–501. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

