Prospective observational study validating the German version of the Control of Allergic Rhinitis and Asthma Test (CARAT10)
- PMID: 30514843
- PMCID: PMC6279774
- DOI: 10.1038/s41533-018-0112-8
Prospective observational study validating the German version of the Control of Allergic Rhinitis and Asthma Test (CARAT10)
Abstract
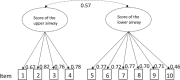
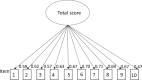
The Control of Allergic Rhinitis and Asthma Test (CARAT10), developed by Portuguese experts, is the only questionnaire assessing asthma control under additional consideration of a frequently concurrent allergic rhinitis (AR), providing sub-scores for both diseases. Aims of this study were the translation and validation of its German version. Asthma patients both with and without AR were included at three primary care pulmologists´ practices in Southern Germany. After translation process, patients completed the CARAT10, the Asthma Control Questionnaire (ACQ), the Asthma Control Test (ACT), and the Standardised Asthma Quality of Life Questionnaire (AQLQ(S)). Item and scale characteristics as well as measures of reliability and validity of the CARAT10 were determined. A confirmatory factor analysis was conducted to test factorial validity. Data of 213 patients were analysed, 101 (47%) of them with concurrent AR. Missing responses were minimal and unsystematic. Cronbach´s α was 0.87 for the CARAT10 total score (TS) and 0.84 for each sub-score. Spearman´s correlation coefficients for the association of the CARAT10 TS with ACQ, ACT and AQLQ(S) were moderate to high and slightly higher in patients with AR. Higher correlations were found for its lower airway sub-score than the upper airway sub-score (all around 0.8 to all around 0.3). Confirmatory factor analysis confirmed the two-factorial scale structure of the CARAT10, with a two-factor model showing a better fit to the data than a one-factor model. The German version of the CARAT10 is an acceptable, reliable and valid tool. Our results suggest a recommended use in asthma patients with AR.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

