A framework for the development of effective anti-metastatic agents
- PMID: 30514977
- PMCID: PMC7136167
- DOI: 10.1038/s41571-018-0134-8
A framework for the development of effective anti-metastatic agents
Abstract
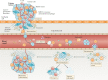
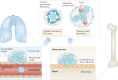
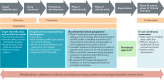
Most cancer-related deaths are a result of metastasis, and thus the importance of this process as a target of therapy cannot be understated. By asking 'how can we effectively treat cancer?', we do not capture the complexity of a disease encompassing >200 different cancer types - many consisting of multiple subtypes - with considerable intratumoural heterogeneity, which can result in variable responses to a specific therapy. Moreover, we have much less information on the pathophysiological characteristics of metastases than is available for the primary tumour. Most disseminated tumour cells that arrive in distant tissues, surrounded by unfamiliar cells and a foreign microenvironment, are likely to die; however, those that survive can generate metastatic tumours with a markedly different biology from that of the primary tumour. To treat metastasis effectively, we must inhibit fundamental metastatic processes and develop specific preclinical and clinical strategies that do not rely on primary tumour responses. To address this crucial issue, Cancer Research UK and Cancer Therapeutics CRC Australia formed a Metastasis Working Group with representatives from not-for-profit, academic, government, industry and regulatory bodies in order to develop recommendations on how to tackle the challenges associated with treating (micro)metastatic disease. Herein, we describe the challenges identified as well as the proposed approaches for discovering and developing anticancer agents designed specifically to prevent or delay the metastatic outgrowth of cancer.
Conflict of interest statement
J.E. has received funding for clinical trials, honoraria, speaker’s fees and support to attend conferences from Bristol Myers Squibb. J.A.H. owns stock in and is an employee and company director of Vivactiv Limited (consultancy). J.A.S. is a member of the Scientific Advisory Board (NGS) for Qiagen and sits on the Council of the International Journal of Experimental Pathology. P.S.S. receives research funding from MedImmune. All authors are employees of their respective organizations and declare no other competing interests.
Figures
References
-
- Spano D, Heck C, De Antonellis P, Christofori G, Zollo M. Molecular networks that regulate cancer metastasis. Semin. Cancer Biol. 2012;22:234–249. - PubMed
-
- Vreeland TJ, et al. Gaining ground on a cure through synergy: combining checkpoint inhibitors with cancer vaccines. Expert Rev. Clin. Immunol. 2016;12:1347–1357. - PubMed
-
- Therasse P, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl Cancer Inst. 2000;92:205–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

