Therapeutic Targeting of IRFs: Pathway-Dependence or Structure-Based?
- PMID: 30515152
- PMCID: PMC6255967
- DOI: 10.3389/fimmu.2018.02622
Therapeutic Targeting of IRFs: Pathway-Dependence or Structure-Based?
Abstract
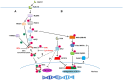
The interferon regulatory factors (IRFs) are a family of master transcription factors that regulate pathogen-induced innate and acquired immune responses. Aberration(s) in IRF signaling pathways due to infection, genetic predisposition and/or mutation, which can lead to increased expression of type I interferon (IFN) genes, IFN-stimulated genes (ISGs), and other pro-inflammatory cytokines/chemokines, has been linked to the development of numerous diseases, including (but not limited to) autoimmune and cancer. What is currently lacking in the field is an understanding of how best to therapeutically target these transcription factors. Many IRFs are regulated by post-translational modifications downstream of pattern recognition receptors (PRRs) and some of these modifications lead to activation or inhibition. We and others have been able to utilize structural features of the IRFs in order to generate dominant negative mutants that inhibit function. Here, we will review potential therapeutic strategies for targeting all IRFs by using IRF5 as a candidate targeting molecule.
Keywords: IRF5; autoimmunity; inhibition; negative regulation; positive regulation.
Figures


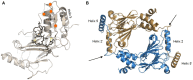
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

