Dysregulated miR-155 and miR-125b Are Related to Impaired B-cell Responses in Down Syndrome
- PMID: 30515165
- PMCID: PMC6255899
- DOI: 10.3389/fimmu.2018.02683
Dysregulated miR-155 and miR-125b Are Related to Impaired B-cell Responses in Down Syndrome
Abstract
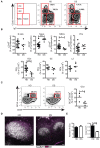
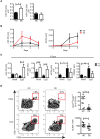
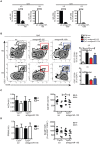
Children with Down Syndrome (DS) suffer from immune deficiency with a severe reduction in switched memory B cells (MBCs) and poor response to vaccination. Chromosome 21 (HSA21) encodes two microRNAs (miRs), miR-125b, and miR-155, that regulate B-cell responses. We studied B- and T- cell subpopulations in tonsils of DS and age-matched healthy donors (HD) and found that the germinal center (GC) reaction was impaired in DS. GC size, numbers of GC B cells and Follicular Helper T cells (TFH) expressing BCL6 cells were severely reduced. The expression of miR-155 and miR-125b was increased in tonsillar memory B cells and miR-125b was also higher than expected in plasma cells (PCs). Activation-induced cytidine deaminase (AID) protein, a miR-155 target, was significantly reduced in MBCs of DS patients. Increased expression of miR-155 was also observed in vitro. MiR-155 was significantly overexpressed in PBMCs activated with CpG, whereas miR-125b was constitutively higher than normal. The increase of miR-155 and its functional consequences were blocked by antagomiRs in vitro. Our data show that the expression of HSA21-encoded miR-155 and miR-125b is altered in B cells of DS individuals both in vivo and in vitro. Because of HSA21-encoded miRs may play a role also in DS-associated dementia and leukemia, our study suggests that antagomiRs may represent pharmacological tools useful for the treatment of DS.
Keywords: B cell; Down Syndrome; antagomiR; germinal center; immunodeficiency; miR-125b; miR-155; plasma cells.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

