Lymphoid Stress Surveillance Response Contributes to Vitiligo Pathogenesis
- PMID: 30515176
- PMCID: PMC6255962
- DOI: 10.3389/fimmu.2018.02707
Lymphoid Stress Surveillance Response Contributes to Vitiligo Pathogenesis
Abstract
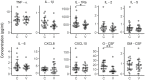
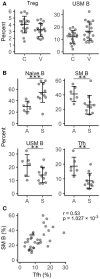
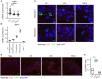
Vitiligo is a chronic multifactorial depigmentation disorder characterized by the destruction and functional loss of melanocytes. Although a direct cytotoxic T cell attack is thought to be responsible for melanocyte damage, the events leading to the loss of self-tolerance toward melanocytic antigens are not understood. This research aimed to identify novel cellular and molecular factors that participate in vitiligo pathogenesis through the application of gene expression and immunofluorescence analysis of skin biopsy samples along with immunophenotyping of circulating cells. Our study provides insights into the mechanisms involved in melanocyte destruction. The upregulation of stress-ligand MICA/MICB, recognized by activating receptors on innate and innate-like T cells, imply involvement of lymphoid stress surveillance responses in vitiligo lesions. A simultaneous increase in the expression of transcription factor EOMES that is characteristic for innate-like virtual memory T cells, suggest a similar scenario. Local lymphoid stress surveillance has been previously associated with the amplification of systemic humoral responses that were mirrored in our study by increased T follicular helper cells and switched memory B cell proportions in patients with active vitiligo. In addition, microtubule-associated protein light chain 3 staining was compatible with the activation of autophagy in keratinocytes and in the remaining melanocytes of vitiligo lesional skin.
Keywords: B cells; EOMES; LC3; MICA/MICB; WIPI1; autophagy; interferons; vitiligo.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

