Kinematical, Structural and Mechanical Adaptations to Desiccation in Poikilohydric Ramonda myconi (Gesneriaceae)
- PMID: 30515187
- PMCID: PMC6256057
- DOI: 10.3389/fpls.2018.01701
Kinematical, Structural and Mechanical Adaptations to Desiccation in Poikilohydric Ramonda myconi (Gesneriaceae)
Abstract
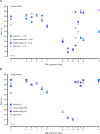
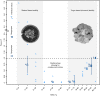
Resurrection plants have fascinated scientists since centuries as they can fully recover from cellular water contents below 10%, concomitantly showing remarkable leaf folding motions. While physiological adaptations have been meticulously investigated, the understanding of structural and mechanical adaptations of this phenomenon is scarce. Using imaging and bending techniques during dehydration-rehydration experiments, morphological, anatomical, and biomechanical properties of desiccation-tolerant Ramonda myconi are examined, and selected structural adaptations are compared to those of homoiohydrous Monophyllaea horsfieldii (both Gesneriaceae). At low water availability, intact and cut-off R. myconi leaves undergo considerable morphological alterations, which are fully and repeatedly reversible upon rehydration. Furthermore, their petioles show a triphasic mechanical behavior having a turgor-based structural stability at high (Phase 1), a flexible mechanically state at intermediate (Phase 2) and a material-based stability at low water contents (Phase 3). Lastly, manipulation experiments with cut-off plant parts revealed that both the shape alterations of individual structures, as well as, the general leaf kinematics largely rely on passive swelling and shrinking processes. Taken together, R. myconi possesses structural and mechanical adaptations to desiccation (in addition to physiological adaptations), which may mainly be passively driven by its water status influenced by the water fluctuations in its surroundings.
Keywords: desiccation tolerance; functional morphology; leaf folding kinematics; plant biomechanics; resurrection plants.
Figures




References
-
- Aloni R. (1987). Differentiation of vascular tissues. Annu. Rev. Plant Physiol. Plant Mol. Biol. 38, 179–204. 10.1146/annurev.pp.38.060187.001143 - DOI
-
- Bateman R. M., Crane P. R., DiMichele W. A., Kenrick P. R., Rowe N. P., Speck T., et al. (1998). Early evolution of land plants: phylogeny, physiology, and ecology of the primary terrestrial radiation. Annu. Rev. Ecol. Evol. Syst. 29, 263–292. 10.1146/annurev.ecolsys.29.1.263 - DOI
LinkOut - more resources
Full Text Sources

