How safe is TDF/FTC as PrEP? A systematic review and meta-analysis of the risk of adverse events in 13 randomised trials of PrEP
- PMID: 30515300
- PMCID: PMC6248833
- DOI: 10.1016/S2055-6640(20)30312-5
How safe is TDF/FTC as PrEP? A systematic review and meta-analysis of the risk of adverse events in 13 randomised trials of PrEP
Abstract
Background: Tenofovir/emtricitabine (TDF/FTC) used as pre-exposure prophylaxis (PrEP) has proven benefits in preventing HIV infection. Widespread use of TDF/FTC can only be justified if the preventative benefits outweigh potential risks of adverse events. A previous meta-analysis of TDF/FTC compared to alternative tenofovir alafenamide (TAF)/FTC for treatment found no significant difference in safety endpoints when used without ritonavir or cobicistat, but more evidence around the safety of TDF/FTC is needed to address concerns and inform widespread use.
Methods: A systematic review identified 13 randomised trials of PrEP, using either TDF/FTC or TDF, versus placebo or no treatment: VOICE, PROUD, IPERGAY, FEM-PrEP, TDF-2, iPrEX, IAVI Kenya, IAVI Uganda, PrEPare, PARTNERS, US Safety study, Bangkok TDF study, W African TDF study. The number of participants with grade 3/4 adverse events or serious adverse events (SAEs) was compared between treatment and control in the meta-analysis. Further analyses of specific renal and bone markers were also undertaken, with fractures as a marker of bone effects and creatinine elevations as a surrogate marker for renal impairment. Analyses were stratified by study duration (</>1 year of follow up).
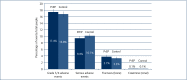
Results: The 13 randomised trials included 15,678 participants in relevant treatment and control arms. Three studies assessed TDF use only. The number of participants with grade 3/4 adverse events was 1306/7504 (17.4%) on treatment versus 1259/7502 (16.8%) on control (difference=0%, 95% confidence interval [CI] -1% to +2%). The number of participants with SAEs was 740/7843 (9.4%) on treatment versus 795/7835 (10.1%) on no treatment (difference=0%, 95% CI -1% to +1%). The number of participants with creatinine elevations was 8/7843 on treatment versus 4/7835 on control (difference=0%, 95% CI 0%-0%). The number of participants with bone fractures was 217/5789 on treatment versus 189/5795 on control (difference=0%, 95% CI 0% to 1%). There was no difference in outcome between studies with <1 versus >1 year of randomised treatment.
Conclusions: In this meta-analysis of 13 randomised clinical trials of PrEP in 15,678 participants, there was no significant difference in risk of grade 3/4 clinical adverse events or SAEs between TDF/FTC (or TDF) and control. Furthermore, there was no significant difference in risk of specific renal or bone adverse outcomes. The favourable safety profile of TDF/FTC would support more widespread use PrEP in populations with a lower risk of HIV infection.
Keywords: Pre-exposure prophylaxis (PrEP), Safety, HIV, tenofovir, emtricitabine, kidney, bone density, adverse events.
Figures





References
-
- UNAIDS UNAIDS Data 2017. Available from: www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_e... ( accessed May 2018).
-
- Rodger A, Cambiano V, Bruun T et al. . Risk of HIV transmission through condomless sex in MSM couples with suppressive ART: the PARTNER2 study extended results in gay men. AIDS 2018. July 2018. Amsterdam, Netherlands. Abstract WEAX0104LB.
-
- Bavinton B, Grinsztejn B, Phanuphak N, Jin F. HIV treatment prevents HIV transmission in male serodiscordant couples in Australia, Thailand and Brazil. IAS 2017. July 2017, Paris, France. Abstract 5469.
-
- World Health Organization HIV/AIDS programme guidance on pre-exposure oral prophylaxis (PrEP) for serodiscordant couples, men and transgender women who have sex with men at high risk of HIV: recommendations for use in the context of demonstration projects. Available at http://apps.who.int/iris/bitstream/handle/10665/75188/9789241503884_eng.... ( accessed May 2018). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
