Evaluation of clinical meaningfulness of estrogen plus progesterone oral capsule (TX-001HR) on moderate to severe vasomotor symptoms
- PMID: 30516713
- PMCID: PMC6493698
- DOI: 10.1097/GME.0000000000001261
Evaluation of clinical meaningfulness of estrogen plus progesterone oral capsule (TX-001HR) on moderate to severe vasomotor symptoms
Abstract
Objective: The aim of this study was to determine the clinical meaningfulness of TX-001HR in reducing moderate to severe vasomotor symptoms (VMS) in menopausal women with a uterus.
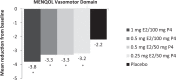
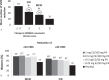
Methods: In the REPLENISH study (NCT01942668), women with moderate to severe hot flushes (≥7/d or ≥50/wk) were enrolled in a VMS substudy and randomized to four doses of daily TX-001HR (17β-estradiol/progesterone) or placebo. Participants assessed improvement of their VMS by the Clinical Global Impression and the Menopause-Specific Quality of Life (MENQOL) questionnaire, which were used to define clinical responders, clinically important differences (CIDs) or minimal CID (MCID) in VMS frequency. Response thresholds were determined by nonparametric discriminant analyses utilizing bootstrapping methods.
Results: In the modified intent-to-treat VMS substudy population (n = 726), statistically significantly more Clinical Global Impression-based clinical responders were observed with TX-001HR than placebo for MCID (weekly reduction of ≥25 moderate to severe VMS: 82-88% vs 69%; all, P < 0.05) and CID (weekly reduction of ≥39 VMS: 68%-73% vs 52%; all, P < 0.05) at week 12. Week 4 results were similar. For Menopause Quality of Life-based analysis, significantly more clinical responders were observed with TX-001HR than placebo for MCID (weekly reduction of ≥34 VMS: 74%-81% vs 55%; all, P < 0.01) and CID (weekly reduction of ≥44 VMS: 61%-69% vs 42%; all, P < 0.01) at week 12.
Conclusions: TX-001HR provided clinically meaningful improvements (as measured by 2 different methods), in addition to statistically significant reductions, in menopausal VMS frequency. TX-001HR may provide a new option, as a single oral capsule of estradiol and progesterone (identical to the hormones naturally occurring in women) for the treatment of moderate to severe VMS in menopausal women with a uterus.
Figures
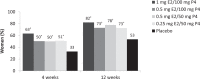

References
-
- Lobo RA, Pinkerton JV, Gass MLS, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic bone parameters and overall safety profile. Fertil Steril 2009; 92:1025–1038. - PubMed
-
- Archer DF, Pickar JH, MacAllister DC, Warren MP. Transdermal estradiol gel for the treatment of symptomatic postmenopausal women. Menopause 2012; 19:622–629. - PubMed
-
- Archer DF, Schmelter T, Schaefers M, Gerlinger C, Gude K. A randomized, double-blind, placebo-controlled study of the lowest effective dose of drospirenone with 17beta-estradiol for moderate to severe vasomotor symptoms in postmenopausal women. Menopause 2014; 21:227–235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

