Efficacy of Prolonged Exposure Therapy, Sertraline Hydrochloride, and Their Combination Among Combat Veterans With Posttraumatic Stress Disorder: A Randomized Clinical Trial
- PMID: 30516797
- PMCID: PMC6439753
- DOI: 10.1001/jamapsychiatry.2018.3412
Efficacy of Prolonged Exposure Therapy, Sertraline Hydrochloride, and Their Combination Among Combat Veterans With Posttraumatic Stress Disorder: A Randomized Clinical Trial
Abstract
Importance: Meta-analyses of treatments for posttraumatic stress disorder (PTSD) suggest that trauma-focused psychotherapies produce greater benefits than antidepressant medications alone.
Objective: To determine the relative efficacy of prolonged exposure therapy plus placebo, prolonged exposure therapy plus sertraline hydrochloride, and sertraline plus enhanced medication management in the treatment of PTSD.
Design, setting, and participants: The Prolonged Exposure and Sertraline Trial was a randomized, multisite, 24-week clinical trial conducted at the Veterans Affairs Ann Arbor Healthcare System, Veterans Affairs San Diego Healthcare System, Ralph H. Johnson Veterans Affairs Medical Center, and Massachusetts General Hospital Home Base Veterans Program between January 26, 2012, and May 9, 2016. Participants and clinicians were blinded to pill condition, and outcome evaluators were blinded to assignment. Participants completed assessments at weeks 0 (intake), 6, 12, 24, and 52 (follow-up). Participants (N = 223) were service members or veterans of the Iraq and/or Afghanistan wars with combat-related PTSD and significant impairment (Clinician-Administered PTSD Scale score, ≥50) of at least 3 months' duration. Analyses were on an intent-to-treat basis.
Intervention: Participants completed up to thirteen 90-minute sessions of prolonged exposure therapy by week 24. Sertraline dosage was titrated during a 10-week period and continued until week 24; medication management was manualized.
Main outcomes and measures: The primary outcome was symptom severity of PTSD in the past month as assessed by the Clinician-Administered PTSD Scale score at week 24.
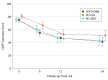
Results: Of 223 randomized participants, 149 completed the study at 24 weeks, and 207 (180 men and 27 women; mean [SD] age, 34.5 [8.3 years]) were included in the intent-to-treat analysis. Modified intent-to-treat analysis using a mixed model of repeated measures showed that PTSD symptoms decreased significantly during the 24 weeks (sertraline plus enhanced medication management, 33.8 points; prolonged exposure therapy plus sertraline, 32.7 points; and prolonged exposure therapy plus placebo, 29.4 points; β,-9.39; 95% CI, -11.62 to -7.16; P < .001); however, slopes did not differ by treatment group (prolonged exposure therapy plus placebo group, -9.39; sertraline plus enhanced medication management group, -10.37; and prolonged exposure therapy plus sertraline group, -9.99; P = .81).
Conclusions and relevance: No difference in change in PTSD symptoms or symptom severity at 24 weeks was found between sertraline plus enhanced medication management, prolonged exposure therapy plus placebo, and prolonged exposure therapy plus sertraline.
Trial registration: ClinicalTrials.gov Identifier: NCT01524133.
Conflict of interest statement
Figures

Comment in
-
First-line Psychotherapies for Military-Related PTSD.JAMA. 2020 Feb 18;323(7):656-657. doi: 10.1001/jama.2019.20825. JAMA. 2020. PMID: 31999301 No abstract available.
References
-
- US Department of Veterans Affairs Management of posttraumatic stress disorder and acute stress reaction 2017. https://www.healthquality.va.gov/guidelines/mh/ptsd/. Updated August 29, 2018. Accessed October 26, 2018.
-
- Institute of Medicine Treatment of PTSD: an assessment of the evidence, report brief. Washington, DC: Institute of Medicine; 2007.
-
- American Psychological Association Clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD). https://www.apa.org/ptsd-guideline/. Accessed October 26, 2018.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

