The Journey of HIV-1 Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) from Lab to Clinic
- PMID: 30516990
- PMCID: PMC7049092
- DOI: 10.1021/acs.jmedchem.8b00843
The Journey of HIV-1 Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) from Lab to Clinic
Abstract
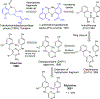
Human immunodeficiency virus (HIV) infection is now pandemic. Targeting HIV-1 reverse transcriptase (HIV-1 RT) has been considered as one of the most successful targets for the development of anti-HIV treatment. Among the HIV-1 RT inhibitors, non-nucleoside reverse transcriptase inhibitors (NNRTIs) have gained a definitive place due to their unique antiviral potency, high specificity, and low toxicity in antiretroviral combination therapies used to treat HIV. Until now, >50 structurally diverse classes of compounds have been reported as NNRTIs. Among them, six NNRTIs were approved for HIV-1 treatment, namely, nevirapine (NVP), delavirdine (DLV), efavirenz (EFV), etravirine (ETR), rilpivirine (RPV), and doravirine (DOR). In this perspective, we focus on the six NNRTIs and lessons learned from their journey through development to clinical studies. It demonstrates the obligatory need of understanding the physicochemical and biological principles (lead optimization), resistance mutations, synthesis, and clinical requirements for drugs.
Conflict of interest statement
CONFLICT OF INTEREST
Victor Kramer is an employee in the medical affairs department of Merck Canada Inc. Kirkland, QC, Canada and previously worked at the McGill AIDS Center/Lady Davis Institute from 2009–2015.
Figures



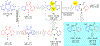


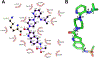

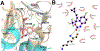






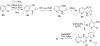
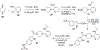
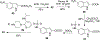
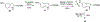

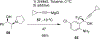



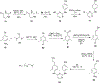
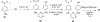


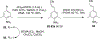
References
-
- Poongavanam V; Namasivayam V; Vanangamudi M; Al Shamaileh H; Veedu RN; Kihlberg J; Murugan NA Integrative approaches in HIV-1 non-nucleoside reverse transcriptase inhibitor design. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2018, 8 (1), e1328.
-
- Zhan P; Pannecouque C; De Clercq E; Liu X Anti-HIV drug discovery and development: Current innovations and future trends. J. Med. Chem. 2016, 59 (7), 2849–78. - PubMed
-
- Mehellou Y; De Clercq E Twenty-six years of anti-HIV drug discovery: where do we stand and where do we go? J. Med. Chem. 2010, 53 (2), 521–538. - PubMed
-
- Flexner C HIV drug development: the next 25 years. Nat. Rev. Drug Discovery 2007, 6 (12), 959–966. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

