Metallaphotoredox-Catalyzed Cross-Electrophile Csp3-Csp3 Coupling of Aliphatic Bromides
- PMID: 30516995
- PMCID: PMC6697083
- DOI: 10.1021/jacs.8b12025
Metallaphotoredox-Catalyzed Cross-Electrophile Csp3-Csp3 Coupling of Aliphatic Bromides
Abstract
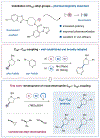
A strategy for the installation of small alkyl fragments onto pharmaceutically relevant aliphatic structures has been established via metallaphotoredox catalysis. Herein, we report that tris(trimethylsilyl)silanol can be employed as an effective halogen abstraction reagent that, in combination with photoredox and nickel catalysis, allows a generic approach to Csp3-Csp3 cross-electrophile coupling. In this study, we demonstrate that a variety of aliphatic drug-like groups can be successfully coupled with a number of commercially available small alkyl electrophiles, including methyl tosylate and strained cyclic alkyl bromides. Moreover, the union of two secondary aliphatic carbon centers, a long-standing challenge for organic molecule construction, has been accomplished with a wide array of structural formats. Last, this technology can be selectively merged with Csp2-Csp3 aryl-alkyl couplings to build drug-like systems in a highly modular fashion.
Figures
References
-
- Lovering F; Bikker J; Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752. - PubMed
-
- Schönherr H; Cernak T Profound Methyl Effects in Drug Discovery and a Call for New C─H Methylation Reactions. Angew. Chem., Int Ed. 2013, 52, 12256. - PubMed
-
- Cox CD; Breslin MJ; Whitman DB; Schreier JD; McGaughey GB; Bogusky MJ; Roecker AJ; Mercer SP; Bednar RA; Lemaire W; Bruno JG; Reiss DR; Harrell CM; Murphy KL; Garson SL; Doran SM; Prueksaritanont T; Anderson WB; Tang C; Roller S; Cabalu TD; Cui D; Hartman GD; Young SD; Koblan KS; Winrow CJ; Renger JJ; Coleman PJ Discovery of the Dual Orexin Receptor Antagonist [(7R)-4-(5-Chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the Treatment of Insomnia. J. Med. Chem 2010, 53, 5320. - PubMed
-
- Devasagayaraj A; Stüdemann T; Knochel P A New Nickel-Catalyzed Cross-Coupling Reaction between sp3 Carbon Centers. Angew. Chem., Int. Ed. Engl 1996, 34, 2723.
- Giovannini R; Stüdemann T; Dussin G; Knochel P An Efficient Nickel-Catalyzed Cross-Coupling Between sp3 Carbon Centers. Angew. Chem., Int. Ed 1998, 37, 2387. - PubMed
-
- Zhou JS; Fu GC Cross-Couplings of Unactivated Secondary Alkyl Halides: Room-Temperature Nickel-Catalyzed Negishi Reactions of Alkyl Bromides and Iodides. J. Am. Chem. Soc. 2003, 125, 14726. - PubMed
- Saito B; Fu GC Alkyl–Alkyl Suzuki Cross-Couplings of Unactivated Secondary Alkyl Halides at Room Temperature. J. Am. Chem. Soc 2007, 129, 9602. - PMC - PubMed
- Cordier CJ; Lundgren RJ; Fu GC Enantioconvergent Cross-Couplings of Racemic Alkylmetal Reagents with Unactivated Secondary Alkyl Electrophiles: Catalytic Asymmetric Negishi α-Alkylations of N-Bocpyrrolidine. J. Am. Chem. Soc 2013, 135, 10946. - PMC - PubMed
- Schmidt J; Choi J; Liu AT; Slusarczyk M; Fu GC A general, modular method for the catalytic asymmetric synthesis of alkylboronate esters. Science 2016, 354, 1265. - PMC - PubMed
- Mu X; Shibata Y; Makida Y; Fu GC Control of Vicinal Stereocenters through Nickel-Catalyzed Alkyl–Alkyl Cross-Coupling. Angew. Chem., Int. Ed 2017, 56, 5821. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources