Ipsilateral finger representations in the sensorimotor cortex are driven by active movement processes, not passive sensory input
- PMID: 30517048
- PMCID: PMC6397402
- DOI: 10.1152/jn.00439.2018
Ipsilateral finger representations in the sensorimotor cortex are driven by active movement processes, not passive sensory input
Abstract
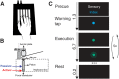
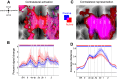
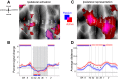
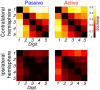
Hand and finger movements are mostly controlled through crossed corticospinal projections from the contralateral hemisphere. During unimanual movements, activity in the contralateral hemisphere is increased while the ipsilateral hemisphere is suppressed below resting baseline. Despite this suppression, unimanual movements can be decoded from ipsilateral activity alone. This indicates that ipsilateral activity patterns represent parameters of ongoing movement, but the origin and functional relevance of these representations is unclear. In this study, we asked whether ipsilateral representations are caused by active movement or whether they are driven by sensory input. Participants alternated between performing single finger presses and having fingers passively stimulated while we recorded brain activity using high-field (7T) functional imaging. We contrasted active and passive finger representations in sensorimotor areas of ipsilateral and contralateral hemispheres. Finger representations in the contralateral hemisphere were equally strong under passive and active conditions, highlighting the importance of sensory information in feedback control. In contrast, ipsilateral finger representations in the sensorimotor cortex were stronger during active presses. Furthermore, the spatial distribution of finger representations differed between hemispheres: the contralateral hemisphere showed the strongest finger representations in Brodmann areas 3a and 3b, whereas the ipsilateral hemisphere exhibited stronger representations in premotor and parietal areas. Altogether, our results suggest that finger representations in the two hemispheres have different origins: contralateral representations are driven by both active movement and sensory stimulation, whereas ipsilateral representations are mainly engaged during active movement. NEW & NOTEWORTHY Movements of the human body are mostly controlled by contralateral cortical regions. The function of ipsilateral activity during movements remains elusive. Using high-field neuroimaging, we investigated how human contralateral and ipsilateral hemispheres represent active and passive finger presses. We found that representations in contralateral sensorimotor cortex are equally strong during both conditions. Ipsilateral representations were mostly present during active movement, suggesting that sensorimotor areas do not receive direct sensory input from the ipsilateral hand.
Keywords: fMRI; finger movements; ipsilateral; motor; sensory.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

