The administration of high-mobility group box 1 fragment prevents deterioration of cardiac performance by enhancement of bone marrow mesenchymal stem cell homing in the delta-sarcoglycan-deficient hamster
- PMID: 30517097
- PMCID: PMC6281303
- DOI: 10.1371/journal.pone.0202838
The administration of high-mobility group box 1 fragment prevents deterioration of cardiac performance by enhancement of bone marrow mesenchymal stem cell homing in the delta-sarcoglycan-deficient hamster
Abstract
Objectives: We hypothesized that systemic administration of high-mobility group box 1 fragment attenuates the progression of myocardial fibrosis and cardiac dysfunction in a hamster model of dilated cardiomyopathy by recruiting bone marrow mesenchymal stem cells thus causing enhancement of a self-regeneration system.
Methods: Twenty-week-old J2N-k hamsters, which are δ-sarcoglycan-deficient, were treated with systemic injection of high-mobility group box 1 fragment (HMGB1, n = 15) or phosphate buffered saline (control, n = 11). Echocardiography for left ventricular function, cardiac histology, and molecular biology were analyzed. The life-prolonging effect was assessed separately using the HMGB1 and control groups, in addition to a monthly HMGB1 group which received monthly systemic injections of high-mobility group box 1 fragment, 3 times (HMGB1, n = 11, control, n = 9, monthly HMGB1, n = 9).
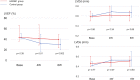
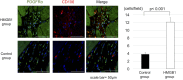
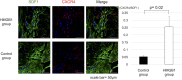
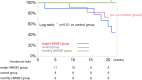
Results: The HMGB1 group showed improved left ventricular ejection fraction, reduced myocardial fibrosis, and increased capillary density. The number of platelet-derived growth factor receptor-alpha and CD106 positive mesenchymal stem cells detected in the myocardium was significantly increased, and intra-myocardial expression of tumor necrosis factor α stimulating gene 6, hepatic growth factor, and vascular endothelial growth factor were significantly upregulated after high-mobility group box 1 fragment administration. Improved survival was observed in the monthly HMGB1 group compared with the control group.
Conclusions: Systemic high-mobility group box 1 fragment administration attenuates the progression of left ventricular remodeling in a hamster model of dilated cardiomyopathy by enhanced homing of bone marrow mesenchymal stem cells into damaged myocardium, suggesting that high-mobility group box 1 fragment could be a new treatment for dilated cardiomyopathy.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures








Similar articles
-
High-mobility group box 1 fragment suppresses adverse post-infarction remodeling by recruiting PDGFRα-positive bone marrow cells.PLoS One. 2020 Apr 10;15(4):e0230392. doi: 10.1371/journal.pone.0230392. eCollection 2020. PLoS One. 2020. PMID: 32275672 Free PMC article.
-
Synthetic prostacyclin agonist, ONO1301, enhances endogenous myocardial repair in a hamster model of dilated cardiomyopathy: a promising regenerative therapy for the failing heart.J Thorac Cardiovasc Surg. 2013 Dec;146(6):1516-25. doi: 10.1016/j.jtcvs.2013.02.045. J Thorac Cardiovasc Surg. 2013. PMID: 24229503
-
Administration of insulin-like growth factor-1 (IGF-1) improves both structure and function of delta-sarcoglycan deficient cardiac muscle in the hamster.Basic Res Cardiol. 2005 Mar;100(2):161-70. doi: 10.1007/s00395-004-0506-3. Epub 2004 Dec 22. Basic Res Cardiol. 2005. PMID: 15611844
-
Pivotal advances: high-mobility group box 1 protein--a cytokine with a role in cardiac repair.J Leukoc Biol. 2007 Jan;81(1):41-5. doi: 10.1189/jlb.0306165. Epub 2006 Aug 29. J Leukoc Biol. 2007. PMID: 16940333 Review.
-
Therapeutic potential of high mobility group box-1 in ischemic injury and tissue regeneration.Curr Vasc Pharmacol. 2011 Nov;9(6):677-81. doi: 10.2174/157016111797484125. Curr Vasc Pharmacol. 2011. PMID: 21692740 Review.
Cited by
-
MicroRNA-381-3p signatures as a diagnostic marker in patients with sepsis and modulates sepsis-steered cardiac damage and inflammation by binding HMGB1.Bioengineered. 2021 Dec;12(2):11936-11946. doi: 10.1080/21655979.2021.2006967. Bioengineered. 2021. PMID: 34784841 Free PMC article.
-
Innovations in the Treatment of Dystrophic Epidermolysis Bullosa (DEB): Current Landscape and Prospects.Ther Clin Risk Manag. 2023 Jun 14;19:455-473. doi: 10.2147/TCRM.S386923. eCollection 2023. Ther Clin Risk Manag. 2023. PMID: 37337559 Free PMC article. Review.
-
High-mobility group box 1 fragment suppresses adverse post-infarction remodeling by recruiting PDGFRα-positive bone marrow cells.PLoS One. 2020 Apr 10;15(4):e0230392. doi: 10.1371/journal.pone.0230392. eCollection 2020. PLoS One. 2020. PMID: 32275672 Free PMC article.
-
Enhancement of tracheal cartilage regeneration by local controlled release of stromal cell-derived factor 1α with gelatin hydrogels and systemic administration of high-mobility group box 1 peptide.Regen Ther. 2024 Jul 4;26:415-424. doi: 10.1016/j.reth.2024.06.017. eCollection 2024 Jun. Regen Ther. 2024. PMID: 39070123 Free PMC article.
-
Investigational Treatments for Epidermolysis Bullosa.Am J Clin Dermatol. 2021 Nov;22(6):801-817. doi: 10.1007/s40257-021-00626-3. Epub 2021 Jul 22. Am J Clin Dermatol. 2021. PMID: 34292508 Review.
References
-
- Weintraub RG, Semsarian C, Macdonald P. Dilated cardiomyopathy. Lancet. 2017;390:400–414. 10.1016/S0140-6736(16)31713-5 - DOI - PubMed
-
- Hoshikawa E, Matsumura Y, Kubo T, Okawa M, Yamasaki N, Kitaoka H, et al. Effect of Left Ventricular Reverse Remodeling on Long-Term Prognosis After Therapy With Angiotensin-Converting Enzyme Inhibitors or Angiotensin II Receptor Blockers and β blockers in Patients With Idiopathic Dilated Cardiomyopathy. Am J Cardiol. 2011;107:1065–1070 10.1016/j.amjcard.2010.11.033 - DOI - PubMed
-
- Kober L, Thune JJ, Nielsen JC, Haarbo J, Videbaek L, Kroup E, et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N Engl J Med. 2016;375:1221–1230 10.1056/NEJMoa1608029 - DOI - PubMed
-
- Ito M, Shinke T, Yoshida A, Kozuki A, Takei A, Fukuzawa K, et al. Reduction in coronary microvascular resistance thorough cardiac resynchronization and its impact on chronic reverse remodeling of left ventricle in patients with non-ischemic cardiomyopathy. Europace. 2015;17:1407–1414 10.1093/europace/euu361 - DOI - PubMed
-
- Dec GW, Fuster V. Idiopathic Dilated Cardiomyopathy. N Engl J Med. 1994;331:1564–1575 10.1056/NEJM199412083312307 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

