Insights on the impact of mitochondrial organisation on bioenergetics in high-resolution computational models of cardiac cell architecture
- PMID: 30517098
- PMCID: PMC6296675
- DOI: 10.1371/journal.pcbi.1006640
Insights on the impact of mitochondrial organisation on bioenergetics in high-resolution computational models of cardiac cell architecture
Abstract
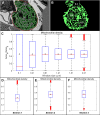
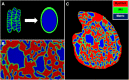
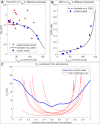
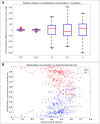
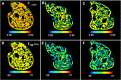
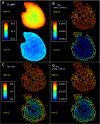
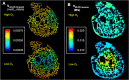
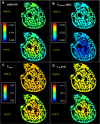
Recent electron microscopy data have revealed that cardiac mitochondria are not arranged in crystalline columns but are organised with several mitochondria aggregated into columns of varying sizes spanning the cell cross-section. This raises the question-how does the mitochondrial arrangement affect the metabolite distributions within cardiomyocytes and what is its impact on force dynamics? Here, we address this question by employing finite element modeling of cardiac bioenergetics on computational meshes derived from electron microscope images. Our results indicate that heterogeneous mitochondrial distributions can lead to significant spatial variation across the cell in concentrations of inorganic phosphate, creatine (Cr) and creatine phosphate (PCr). However, our model predicts that sufficient activity of the creatine kinase (CK) system, coupled with rapid diffusion of Cr and PCr, maintains near uniform ATP and ADP ratios across the cell cross sections. This homogenous distribution of ATP and ADP should also evenly distribute force production and twitch duration with contraction. These results suggest that the PCr shuttle and associated enzymatic reactions act to maintain uniform force dynamics in the cell despite the heterogeneous mitochondrial organization. However, our model also predicts that under hypoxia activity of mitochondrial CK enzymes and diffusion of high-energy phosphate compounds may be insufficient to sustain uniform ATP/ADP distribution and hence force generation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Bittl JA, Ingwall JS. Reaction rates of creatine kinase and ATP synthesis in the isolated rat heart. A 31P NMR magnetization transfer study. Journal of Biological Chemistry. 1985;260(6):3512–7. - PubMed
-
- Katz LA, Swain JA, Portman MA, Balaban RS. Relation between phosphate metabolites and oxygen consumption of heart in vivo. American Journal of Physiology-Heart and Circulatory Physiology. 1989;256(1):H265–H74. 10.1152/ajpheart.1989.256.1.H265 . - DOI - PubMed
-
- Williamson JR, Ford C, Illingworth J, Safer B. Coordination of citric acid cycle activity with electron transport flux. Circulation research. 1976;38(5 Suppl 1):I39–I51. - PubMed
-
- Vendelin M, Béraud N, Guerrero K, Andrienko T, Kuznetsov AV, Olivares J, et al. Mitochondrial regular arrangement in muscle cells: a “crystal-like” pattern. American Journal of Physiology—Cell Physiology. 2005;288(3):C757–C67. 10.1152/ajpcell.00281.2004 - DOI - PubMed
-
- Birkedal R, Shiels HA, Vendelin M. Three-dimensional mitochondrial arrangement in ventricular myocytes: from chaos to order. American Journal of Physiology—Cell Physiology. 2006;291(6):C1148–C58. 10.1152/ajpcell.00236.2006 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

