Direct CNS delivery of proteins using thermosensitive liposome-in-gel carrier by heterotopic mucosal engrafting
- PMID: 30517163
- PMCID: PMC6281301
- DOI: 10.1371/journal.pone.0208122
Direct CNS delivery of proteins using thermosensitive liposome-in-gel carrier by heterotopic mucosal engrafting
Abstract
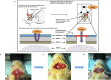
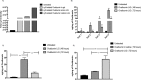
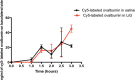
Delivering therapeutics across the blood-brain barrier (BBB) for treating central nervous system (CNS) diseases is one of the biggest challenges today as the BBB limits the uptake of molecules greater than 500 Da into the CNS. Here we describe a novel trans-nasal mucosal drug delivery as an alternative to the intranasal drug delivery to overcome its limitations and deliver high molecular weight (HMW) therapeutics efficiently to the brain. This approach is based on human endoscopic skull base surgical techniques in which a surgical defect is repaired by engrafting semipermeable nasal mucosa over a skull base defect. Based on endoscopic skull based surgeries, our groups has developed a trans-nasal mucosal rodent model where we have evaluated the permeability of ovalbumin (45 kDa) as a model protein through the implanted mucosal graft for delivering HMW therapeutics to the brain. A thermo sensitive liposome-in-gel (LiG) system was developed for creating a drug depot allowing for a sustained release from the site of delivery to the brain through the implanted nasal graft. We would like to report this as an exploratory pilot study where we are using this novel surgical model to show that the implanted nasal mucosal graft and the LiG delivery system result in an efficient and a sustained brain delivery of HMW proteins. Hence, this study demonstrates that the trans-nasal mucosal engrafting technique could overcome the limitations for intranasal drug delivery and enable the uptake of HMW protein therapeutics into the CNS for the treatment of a wide range of neurodegenerative diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Permeabilization of the blood-brain barrier via mucosal engrafting: implications for drug delivery to the brain.PLoS One. 2013 Apr 24;8(4):e61694. doi: 10.1371/journal.pone.0061694. Print 2013. PLoS One. 2013. PMID: 23637885 Free PMC article.
-
Heterotopic mucosal engrafting procedure for direct drug delivery to the brain in mice.J Vis Exp. 2014 Jul 16;(89):51452. doi: 10.3791/51452. J Vis Exp. 2014. PMID: 25077554 Free PMC article.
-
Minimally invasive nasal infusion (MINI) approach for CNS delivery of protein therapeutics: A case study with ovalbumin.J Control Release. 2024 Aug;372:674-681. doi: 10.1016/j.jconrel.2024.06.056. Epub 2024 Jul 1. J Control Release. 2024. PMID: 38909700
-
The blood-brain barrier and nasal drug delivery to the central nervous system.Am J Rhinol Allergy. 2015 Mar-Apr;29(2):124-7. doi: 10.2500/ajra.2015.29.4149. Am J Rhinol Allergy. 2015. PMID: 25785753 Review.
-
Intranasal Drug Delivery: A Non-Invasive Approach for the Better Delivery of Neurotherapeutics.Pharm Nanotechnol. 2017;5(3):203-214. doi: 10.2174/2211738505666170515113936. Pharm Nanotechnol. 2017. PMID: 28521670 Review.
Cited by
-
Scope and challenges of nanoparticle-based mRNA delivery in cancer treatment.Arch Pharm Res. 2022 Dec;45(12):865-893. doi: 10.1007/s12272-022-01418-x. Epub 2022 Nov 24. Arch Pharm Res. 2022. PMID: 36422795 Free PMC article. Review.
-
Editorial: Neuroprotection and Disease Modification in Parkinson's Disease.Front Pharmacol. 2021 Dec 8;12:813471. doi: 10.3389/fphar.2021.813471. eCollection 2021. Front Pharmacol. 2021. PMID: 34955865 Free PMC article. No abstract available.
-
Minimally Invasive Nasal Depot (MIND) technique for direct BDNF AntagoNAT delivery to the brain.J Control Release. 2021 Mar 10;331:176-186. doi: 10.1016/j.jconrel.2021.01.027. Epub 2021 Jan 21. J Control Release. 2021. PMID: 33484777 Free PMC article.
-
Oleanolic Acid Cubic Liquid Crystal Nanoparticle-Based Thermosensitive Gel Attenuates Depression Symptoms in Chronic Unpredictable Mild Stress Rats.Drug Des Devel Ther. 2025 Feb 1;19:715-736. doi: 10.2147/DDDT.S484567. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 39911446 Free PMC article.
-
Recent Advances in Intranasal Liposomes for Drug, Gene, and Vaccine Delivery.Pharmaceutics. 2023 Jan 6;15(1):207. doi: 10.3390/pharmaceutics15010207. Pharmaceutics. 2023. PMID: 36678838 Free PMC article. Review.
References
-
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013 10.1038/nrdp.2017.13 . - DOI - PubMed
-
- Gitler AD, Dhillon P, Shorter J. Neurodegenerative disease: models, mechanisms, and a new hope. Dis Model Mech. 2017;10(5):499–502. 10.1242/dmm.030205 ; PubMed Central PMCID: PMCPMC5451177. - DOI - PMC - PubMed
-
- Noble W, Burns MP. Challenges in neurodegeneration research. Frontiers in psychiatry. 2010;1:7 Epub 2010/01/01. 10.3389/fpsyt.2010.00007 ; PubMed Central PMCID: PMC3059656. - DOI - PMC - PubMed
-
- Oliveira SL, Pillat MM, Cheffer A, Lameu C, Schwindt TT, Ulrich H. Functions of neurotrophins and growth factors in neurogenesis and brain repair. Cytometry A. 2013;83(1):76–89. 10.1002/cyto.a.22161 . - DOI - PubMed
-
- Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer's disease. Nature. 2016;537(7618):50–6. 10.1038/nature19323 . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

