Comparative effectiveness and safety of interventions for acute diarrhea and gastroenteritis in children: A systematic review and network meta-analysis
- PMID: 30517196
- PMCID: PMC6281220
- DOI: 10.1371/journal.pone.0207701
Comparative effectiveness and safety of interventions for acute diarrhea and gastroenteritis in children: A systematic review and network meta-analysis
Abstract
Background: Many interventions have shown effectiveness in reducing the duration of acute diarrhea and gastroenteritis (ADG) in children. Yet, there is lack of comparative efficacy of interventions that seem to be better than placebo among which, the clinicians must choose. Our aim was to determine the comparative effectiveness and safety of the pharmacological and nutritional interventions for reducing the duration of ADG in children.
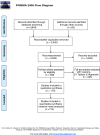
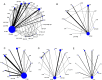
Methods: Data sources included Medline, Embase, CENTRAL, CINAHL, LILACS, and Global-Health up to May 2017. Eligible trials compared zinc (ZN), vitamin A, micronutrients (MN), probiotics, prebiotics, symbiotics, racecadotril, smectite(SM), loperamide, diluted milk, lactose-free formula(LCF), or their combinations, to placebo or standard treatment (STND), or among them. Two reviewers independently performed screening, review, study selection and extraction. The primary outcome was diarrhea duration. Secondary outcomes were stool frequency at day 2, diarrhea at day 3, vomiting and side effects. We performed a random effects Bayesian network meta-analysis to combine the direct and indirect evidence for each outcome. Mean differences and odds ratio with their credible intervals(CrI) were calculated. Coherence and transitivity assumptions were assessed. Meta-regression, subgroups and sensitivity analyses were conducted to explore the impact of effect modifiers. Summary under the cumulative curve (SUCRA) values with their CrI were calculated. We assessed the evidence quality and classified the best interventions using the Grading of Recommendations, Assessment, Development & Evaluation (GRADE) approach for each paired comparison.
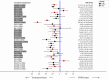
Results: A total of 174 studies (32,430 children) proved eligible. Studies were conducted in 42 countries of which most were low-and middle-income countries (LMIC). Interventions were grouped in 27 categories. Most interventions were better than STND. Reduction of diarrhea varied from 12.5 to 51.1 hours. The combinations Saccharomyces boulardii (SB)+ZN, and SM+ZN were considered the best interventions (i.e., GRADE quality of evidence: moderate to high, substantial superiority to STND, reduction in duration of 35 to 40 hours, and large SUCRA values), while symbiotics (combination of probiotics+prebiotics), ZN, loperamide and combinations ZN+MN and ZN+LCF were considered inferior to the best and better than STND [Quality: moderate to high, superior to STND, and reduction of 17 to 25 hours]. In subgroups analyses, effect of ZN was higher in LMIC and was not present in high-income countries (HIC). Vitamin A, MN, prebiotics, kaolin-pectin, and diluted milk were similar to STND [Quality: moderate to high]. The remainder of the interventions had low to very-low evidence quality. Loperamide was the only intervention with more side effects than STND [Quality: moderate].
Discussion/conclusion: Most interventions analyzed (except vitamin A, micronutrients, prebiotics, and kaolin-pectin) showed evidence of superiority to placebo in reducing the diarrhea. With moderate-to high-quality of evidence, SB+ZN and SM+ZN, demonstrated the best combination of evidence quality and magnitude of effect while symbiotics, loperamide and zinc proved being the best single interventions, and loperamide was the most unsafe. Nonetheless, the effect of zinc, SB+ZN and SM+ZN might only be applied to children in LMIC. Results suggest no further role for studies comparing interventions against no treatment or placebo, or studies testing loperamide, MN, kaolin-pectin, vitamin A, prebiotics and diluted milk.
Prospero registration: CRD42015023778.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? The Lancet. 2003;361(9376):2226–34. - PubMed
-
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. The Lancet. 2015;385(9966):430–40. - PubMed
-
- Schnadower D, Finkelstein Y, Freedman SB. Ondansetron and probiotics in the management of pediatric acute gastroenteritis in developed countries. Current Opinion in Gastroenterology. 2015;31(1):1–6. 10.1097/MOG.0000000000000132 00001574-201501000-00002. - DOI - PubMed
-
- WHO. The treatment of diarrhoea: a manual for physicians and other senior health workers. In: Organization WH, editor. 2005.
-
- American Academy of Pediatrics A. Practice Parameter: The Management of Acute Gastroenteritis in Young Children. Pediatrics. 1996;97(3):424–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

