Vectored delivery of anti-SIV envelope targeting mAb via AAV8 protects rhesus macaques from repeated limiting dose intrarectal swarm SIVsmE660 challenge
- PMID: 30517201
- PMCID: PMC6296672
- DOI: 10.1371/journal.ppat.1007395
Vectored delivery of anti-SIV envelope targeting mAb via AAV8 protects rhesus macaques from repeated limiting dose intrarectal swarm SIVsmE660 challenge
Abstract
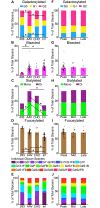
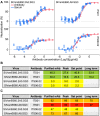
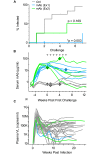
Gene based delivery of immunoglobulins promises to safely and durably provide protective immunity to individuals at risk of acquiring infectious diseases such as HIV. We used a rhesus macaque animal model to optimize delivery of naturally-arising, autologous anti-SIV neutralizing antibodies expressed by Adeno-Associated Virus 8 (AAV8) vectors. Vectored transgene expression was confirmed by quantitation of target antibody abundance in serum and mucosal surfaces. We tested the expression achieved at varying doses and numbers of injections. Expression of the transgene reached a saturation at about 2 x 10(12) AAV8 genome copies (gc) per needle-injection, a physical limitation that may not scale clinically into human trials. In contrast, expression increased proportionately with the number of injections. In terms of anti-drug immunity, anti-vector antibody responses were universally strong, while those directed against the natural transgene mAb were detected in only 20% of animals. An anti-transgene antibody response was invariably associated with loss of detectable plasma expression of the antibody. Despite having atypical glycosylation profiles, transgenes derived from AAV-directed muscle cell expression retained full functional activity, including mucosal accumulation, in vitro neutralization, and protection against repeated limiting dose SIVsmE660 swarm challenge. Our findings demonstrate feasibility of a gene therapy-based passive immunization strategy against infectious disease, and illustrate the potential for the nonhuman primate model to inform clinical AAV-based approaches to passive immunization.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
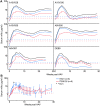
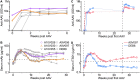
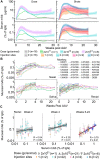
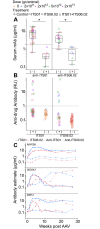
References
-
- Walker LM, Burton DR. Passive immunotherapy of viral infections: “super-antibodies” enter the fray. Nat Rev Immunol. Nature Publishing Group; 2018; 10.1038/nri.2017.148 - DOI - PMC - PubMed
-
- Marston HD, Paules CI, Fauci AS. Monoclonal Antibodies for Emerging Infectious Diseases—Borrowing from History. N Engl J Med. 2018; 1–4. 10.1056/NEJMp1802256 - DOI - PubMed
-
- Salazar G, Zhang N, Fu T-M, An Z. Antibody therapies for the prevention and treatment of viral infections. NPJ vaccines. Springer US; 2017;2: 19 10.1038/s41541-017-0019-3 - DOI - PMC - PubMed
-
- Kelley B. Industrialization of mAb production technology: the bioprocessing industry at a crossroads. MAbs. 2009;1: 443–52. 10.4161/mabs.1.5.9448 - DOI - PMC - PubMed
-
- Shukla AA, Wolfe LS, Mostafa SS, Norman C. Evolving trends in mAb production processes. Bioeng Transl Med. 2017;2: 58–69. 10.1002/btm2.10061 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

