Persistence of Lassa Virus Associated With Severe Systemic Arteritis in Convalescing Guinea Pigs (Cavia porcellus)
- PMID: 30517671
- PMCID: PMC6500557
- DOI: 10.1093/infdis/jiy641
Persistence of Lassa Virus Associated With Severe Systemic Arteritis in Convalescing Guinea Pigs (Cavia porcellus)
Abstract
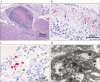
Lassa fever (LF) survivors develop various clinical manifestations including polyserositis, myalgia, epididymitis, and hearing loss weeks to months after recovery from acute infection. We demonstrate a systemic lymphoplasmacytic and histiocytic arteritis and periarteritis in guinea pigs more than 2 months after recovery from acute Lassa virus (LASV) infection. LASV was detected in the arterial tunica media smooth muscle cells by immunohistochemistry, in situ hybridization, and transmission electron microscopy. Our results suggest that the sequelae of LASV infection may be due to virus persistence resulting in systemic vascular damage. These findings shed light on the pathogenesis of LASV sequelae in convalescent human survivors.
Keywords: Arenaviridae; BSL-4; Lassa virus persistent infection; arenavirus; arteritis; guinea pig; mammarenavirus; periarteritis; viral hemorrhagic fever.
Published by Oxford University Press for the Infectious Diseases Society of America 2018.
Figures