HDAC3 Regulates the Transition to the Homeostatic Myelinating Schwann Cell State
- PMID: 30517863
- PMCID: PMC6293966
- DOI: 10.1016/j.celrep.2018.11.045
HDAC3 Regulates the Transition to the Homeostatic Myelinating Schwann Cell State
Abstract
The formation of myelinating Schwann cells (mSCs) involves the remarkable biogenic process, which rapidly generates the myelin sheath. Once formed, the mSC transitions to a stable homeostatic state, with loss of this stability associated with neuropathies. The histone deacetylases histone deacetylase 1 (HDAC1) and HDAC2 are required for the myelination transcriptional program. Here, we show a distinct role for HDAC3, in that, while dispensable for the formation of mSCs, it is essential for the stability of the myelin sheath once formed-with loss resulting in progressive severe neuropathy in adulthood. This is associated with the prior failure to downregulate the biogenic program upon entering the homeostatic state leading to hypertrophy and hypermyelination of the mSCs, progressing to the development of severe myelination defects. Our results highlight distinct roles of HDAC1/2 and HDAC3 in controlling the differentiation and homeostatic states of a cell with broad implications for the understanding of this important cell-state transition.
Keywords: HDACs; Schwann cells; biogenesis; homeostasis; neuropathy; peripheral nerve.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

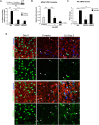
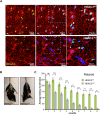


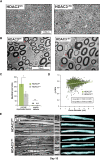

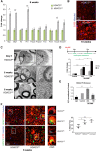
References
-
- Bolis A., Coviello S., Bussini S., Dina G., Pardini C., Previtali S.C., Malaguti M., Morana P., Del Carro U., Feltri M.L. Loss of Mtmr2 phosphatase in Schwann cells but not in motor neurons causes Charcot-Marie-Tooth type 4B1 neuropathy with myelin outfoldings. J. Neurosci. 2005;25:8567–8577. - PMC - PubMed
-
- Brügger V., Engler S., Pereira J.A., Ruff S., Horn M., Welzl H., Münger E., Vaquié A., Sidiropoulos P.N., Egger B. HDAC1/2-dependent P0 expression maintains paranodal and nodal integrity independently of myelin stability through interactions with neurofascins. PLoS Biol. 2015;13:e1002258. - PMC - PubMed
-
- Camargo N., Smit A.B., Verheijen M.H.G. SREBPs: SREBP function in glia-neuron interactions. FEBS J. 2009;276:628–636. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

