Evaluation of Continuous Lactate Monitoring Systems within a Heparinized In Vivo Porcine Model Intravenously and Subcutaneously
- PMID: 30518105
- PMCID: PMC6316727
- DOI: 10.3390/bios8040122
Evaluation of Continuous Lactate Monitoring Systems within a Heparinized In Vivo Porcine Model Intravenously and Subcutaneously
Abstract
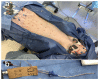
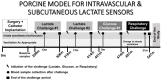
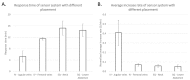
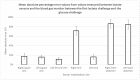
We present an animal model used to evaluate the in vivo performance of electrochemical amperometric continuous lactate sensors compared to blood gas instruments. Electrochemical lactate sensors were fabricated, placed into 5 Fr central venous catheters (CVCs), and paired with wireless potentiostat devices. Following in vivo evaluation and calibration, sensors were placed within the jugular and femoral veins of a porcine subject as a preliminary assessment of in vivo measurement accuracy. The mobile electronic circuit potentiostat devices supplied the operational voltage for the sensors, measured the resultant steady-state current, and recorded the sensor response values in internal memory storages. An in vivo time trace of implanted intravenous (IV) sensors demonstrated lactate values that correlated well with the discrete measurements of blood samples on a benchtop point-of-care sensor-based instrument. Currents measured continuously from the implanted lactate sensors over 10 h were converted into lactate concentration values through use of a two-point in vivo calibration. Study shows that intravenously implanted sensors had more accurate readings, faster peak-reaching rates, and shorter peak-detection times compared to subcutaneously placed sensors. IV implanted and subcutaneously placed sensors closer to the upper body (in this case neck) showed faster response rates and more accurate measurements compared to those implanted in the lower portion of the porcine model. This study represents an important milestone not only towards continuous lactate monitoring for early diagnosis and intervention in neonatal patients with congenital heart disease undergoing cardiopulmonary bypass surgeries, but also in the intervention of critical ill patients in the Intensive Care Units or during complex surgical procedures.
Keywords: cardiopulmonary bypass surgeries; congenital heart disease; continuous blood lactate monitoring; intravenous; lactate sensors; subcutaneous.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Evaluation of an Anti-Thrombotic Continuous Lactate and Blood Pressure Monitoring Catheter in an In Vivo Piglet Model undergoing Open-Heart Surgery with Cardiopulmonary Bypass.Chemosensors (Basel). 2020 Sep;8(3):56. doi: 10.3390/chemosensors8030056. Epub 2020 Jul 17. Chemosensors (Basel). 2020. PMID: 35310780 Free PMC article.
-
Evaluation of subcutaneously-implanted glucose sensors for continuous glucose measurements in hyperglycemic pigs.In Vivo. 2006 Mar-Apr;20(2):195-203. In Vivo. 2006. PMID: 16634519
-
Wearable multiplexed biosensor system toward continuous monitoring of metabolites.Biosens Bioelectron. 2020 Apr 1;153:112038. doi: 10.1016/j.bios.2020.112038. Epub 2020 Jan 18. Biosens Bioelectron. 2020. PMID: 31989942
-
Lactate biosensors for continuous monitoring.Front Biosci. 2004 Sep 1;9:3384-91. doi: 10.2741/1489. Front Biosci. 2004. PMID: 15353365 Review.
-
Performance of subcutaneously implanted glucose sensors: a review.J Invest Surg. 1998 May-Jun;11(3):163-74. doi: 10.3109/08941939809098031. J Invest Surg. 1998. PMID: 9743484 Review.
Cited by
-
Evaluation of an Anti-Thrombotic Continuous Lactate and Blood Pressure Monitoring Catheter in an In Vivo Piglet Model undergoing Open-Heart Surgery with Cardiopulmonary Bypass.Chemosensors (Basel). 2020 Sep;8(3):56. doi: 10.3390/chemosensors8030056. Epub 2020 Jul 17. Chemosensors (Basel). 2020. PMID: 35310780 Free PMC article.
-
Real-Time, In Vivo Molecular Monitoring Using Electrochemical Aptamer Based Sensors: Opportunities and Challenges.ACS Sens. 2022 Oct 28;7(10):2823-2832. doi: 10.1021/acssensors.2c01428. Epub 2022 Oct 7. ACS Sens. 2022. PMID: 36205360 Free PMC article. Review.
-
Monitoring with In Vivo Electrochemical Sensors: Navigating the Complexities of Blood and Tissue Reactivity.Sensors (Basel). 2020 Jun 2;20(11):3149. doi: 10.3390/s20113149. Sensors (Basel). 2020. PMID: 32498360 Free PMC article. Review.
References
-
- Jansen T.C., van Bommel J., Schoonderbeek F.J., Sleeswijk Visser S.J., van der Klooster J.M., Lima A.P., Willemsen S.P., Bakker J. Early lactate-guided therapy in intensive care unit patients: A multicenter, open-label, randomized controlled trial. Am. J. Respir. Crit. Care Med. 2010;182:752–761. doi: 10.1164/rccm.200912-1918OC. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

