Fluorescently Tagged CCL19 and CCL21 to Monitor CCR7 and ACKR4 Functions
- PMID: 30518137
- PMCID: PMC6321256
- DOI: 10.3390/ijms19123876
Fluorescently Tagged CCL19 and CCL21 to Monitor CCR7 and ACKR4 Functions
Abstract
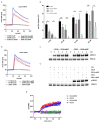
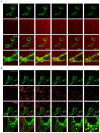
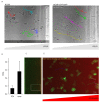
Chemokines are essential guidance cues orchestrating cell migration in health and disease. Cognate chemokine receptors sense chemokine gradients over short distances to coordinate directional cell locomotion. The chemokines CCL19 and CCL21 are essential for recruiting CCR7-expressing dendritic cells bearing pathogen-derived antigens and lymphocytes to lymph nodes, where the two cell types meet to launch an adaptive immune response against the invading pathogen. CCR7-expressing cancer cells are also recruited by CCL19 and CCL21 to metastasize in lymphoid organs. In contrast, atypical chemokine receptors (ACKRs) do not transmit signals required for cell locomotion but scavenge chemokines. ACKR4 is crucial for internalizing and degrading CCL19 and CCL21 to establish local gradients, which are sensed by CCR7-expressing cells. Here, we describe the production of fluorescently tagged chemokines by fusing CCL19 and CCL21 to monomeric red fluorescent protein (mRFP). We show that purified CCL19-mRFP and CCL21-mRFP are versatile and powerful tools to study CCR7 and ACKR4 functions, such as receptor trafficking and chemokine scavenging, in a spatiotemporal fashion. We demonstrate that fluorescently tagged CCL19 and CCL21 permit the visualization and quantification of chemokine gradients in real time, while CCR7-expressing leukocytes and cancer cells sense the guidance cues and migrate along the chemokine gradients.
Keywords: ACKR4; CCL19; CCL21; CCR7; cancer metastasis; cell migration; chemokine receptors; fluorescent chemokines; leukocytes.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
MeSH terms
Substances
Grants and funding
- 31003A_169936/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
- CRSII3_160719/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
- BITg/Staatssekretariat für Bildung, Forschung und Innovation
- BITg/Thurgauische Stiftung für Wissenschaft und Forschung
LinkOut - more resources
Full Text Sources

