Villainous role of estrogen in macrophage-nerve interaction in endometriosis
- PMID: 30518376
- PMCID: PMC6282253
- DOI: 10.1186/s12958-018-0441-z
Villainous role of estrogen in macrophage-nerve interaction in endometriosis
Abstract
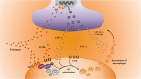
Endometriosis is a complex and heterogeneous disorder with unknown etiology. Dysregulation of macrophages and innervation are important factors influencing the pathogenesis of endometriosis-associated pain. It is known to be an estrogen-dependent disease, estrogen can promote secretion of chemokines from peripheral nerves, enhancing the recruitment and polarization of macrophages in endometriotic tissue. Macrophages have a role in the expression of multiple nerve growth factors (NGF), which mediates the imbalance of neurogenesis in an estrogen-dependent manner. Under the influence of estrogen, co-existence of macrophages and nerves induces an innovative neuro-immune communication. Persistent stimulation by inflammatory cytokines from macrophages on nociceptors of peripheral nerves aggravates neuroinflammation through the release of inflammatory neurotransmitters. This neuro-immune interaction regulated by estrogen sensitizes peripheral nerves, leading to neuropathic pain in endometriosis. The aim of this review is to highlight the significance of estrogen in the interaction between macrophages and nerve fibers, and to suggest a potentially valuable therapeutic target for endometriosis-associated pain.
Keywords: Endometriosis; Estrogen; Macrophage; Nerve fiber; Neuroinflammation.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

