Alzheimer's Disease Risk Factor Pyk2 Mediates Amyloid-β-Induced Synaptic Dysfunction and Loss
- PMID: 30518596
- PMCID: PMC6343652
- DOI: 10.1523/JNEUROSCI.1873-18.2018
Alzheimer's Disease Risk Factor Pyk2 Mediates Amyloid-β-Induced Synaptic Dysfunction and Loss
Abstract
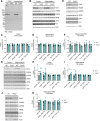
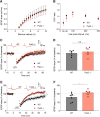
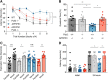
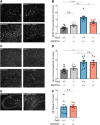
Dozens of genes have been implicated in late onset Alzheimer's disease (AD) risk, but none has a defined mechanism of action in neurons. Here, we show that the risk factor Pyk2 (PTK2B) localizes specifically to neurons in adult brain. Absence of Pyk2 has no major effect on synapse formation or the basal parameters of synaptic transmission in the hippocampal Schaffer collateral pathway. However, the induction of synaptic LTD is suppressed in Pyk2-null slices. In contrast, deletion of Pyk2 expression does not alter LTP under control conditions. Of relevance for AD pathophysiology, Pyk2-/- slices are protected from amyloid-β-oligomer (Aβo)-induced suppression of LTP in hippocampal slices. Acutely, a Pyk2 kinase inhibitor also prevents Aβo-induced suppression of LTP in WT slices. Female and male transgenic AD model mice expressing APPswe/PSEN1ΔE9 require Pyk2 for age-dependent loss of synaptic markers and for impairment of learning and memory. However, absence of Pyk2 does not alter Aβ accumulation or gliosis. Therefore, the Pyk2 risk gene is directly implicated in a neuronal Aβo signaling pathway impairing synaptic anatomy and function.SIGNIFICANCE STATEMENT Genetic variation at the Pyk2 (PTK2B) locus is a risk for late onset Alzheimer's disease (AD), but the pathophysiological role of Pyk2 is not clear. Here, we studied Pyk2 neuronal function in mice lacking expression with and without transgenes generating amyloid-β (Aβ) plaque pathology. Pyk2 is not required for basal synaptic transmission or LTP, but participates in LTD. Hippocampal slices lacking Pyk2 are protected from AD-related Aβ oligomer suppression of synaptic plasticity. In transgenic AD model mice, deletion of Pyk2 rescues synaptic loss and learning/memory deficits. Therefore, Pyk2 plays a central role in AD-related synaptic dysfunction mediating Aβ-triggered dysfunction.
Keywords: Alzheimer's; Pyk2; amyloid; synapse.
Copyright © 2019 the authors 0270-6474/19/390758-15$15.00/0.
Figures




References
-
- Andreev J, Galisteo ML, Kranenburg O, Logan SK, Chiu ES, Okigaki M, Cary LA, Moolenaar WH, Schlessinger J (2001) Src and Pyk2 mediate G-protein-coupled receptor activation of epidermal growth factor receptor (EGFR) but are not required for coupling to the mitogen-activated protein (MAP) kinase signaling cascade. J Biol Chem 276:20130–20135. 10.1074/jbc.M102307200 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
