Peptidylarginine deiminases 2 and 4 modulate innate and adaptive immune responses in TLR-7-dependent lupus
- PMID: 30518690
- PMCID: PMC6328098
- DOI: 10.1172/jci.insight.124729
Peptidylarginine deiminases 2 and 4 modulate innate and adaptive immune responses in TLR-7-dependent lupus
Abstract
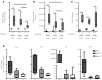
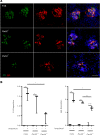
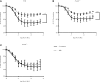
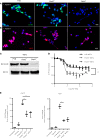
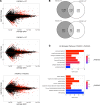
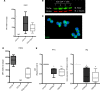
The peptidylarginine deiminases PAD2 and PAD4 are implicated in the pathogenesis of several autoimmune diseases. PAD4 may be pathogenic in systemic lupus erythematosus (SLE) through its role in neutrophil extracellular trap (NET) formation that promotes autoantigen externalization, immune dysregulation, and organ damage. The role of this enzyme in mouse models of autoimmunity remains unclear, as pan-PAD chemical inhibitors improve clinical phenotype, whereas PAD4-KO models have given conflicting results. The role of PAD2 in SLE has not been investigated. The differential roles of PAD2 and PAD4 in TLR-7-dependent lupus autoimmunity were examined. Padi4-/- displayed decreased autoantibodies, type I IFN responses, immune cell activation, vascular dysfunction, and NET immunogenicity. Padi2-/- mice showed abrogation of Th subset polarization, with some disease manifestations reduced compared with WT but to a lesser extent than Padi4-/- mice. RNA sequencing analysis revealed distinct modulation of immune-related pathways in PAD-KO lymphoid organs. Human T cells express both PADs and, when exposed to either PAD2 or PAD4 inhibitors, displayed abrogation of Th1 polarization. These results suggest that targeting PAD2 and/or PAD4 activity modulates dysregulated TLR-7-dependent immune responses in lupus through differential effects of innate and adaptive immunity. Compounds that target PADs may have potential therapeutic roles in T cell-mediated diseases.
Keywords: Autoimmune diseases; Autoimmunity; Inflammation; Lupus; Neutrophils.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

