Extracellular Protein Fibulin-7 and Its C-Terminal Fragment Have In Vivo Antiangiogenic Activity
- PMID: 30518776
- PMCID: PMC6281620
- DOI: 10.1038/s41598-018-36182-w
Extracellular Protein Fibulin-7 and Its C-Terminal Fragment Have In Vivo Antiangiogenic Activity
Abstract
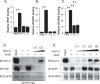
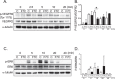
Angiogenesis is crucial for tissue development and homeostasis; however, excessive angiogenesis can lead to diseases, including arthritis and cancer metastasis. Some antiangiogenic drugs are available, but side effects remain problematic. Thus, alternative angiogenesis inhibition strategies are needed. Fibulin-7 (Fbln7) is a newly discovered member of the fibulin protein family, a group of cell-secreted glycoproteins, that functions as a cell adhesion molecule and interacts with other extracellular matrix (ECM) proteins as well as cell receptors. We previously showed that a recombinant C-terminal Fbln7 fragment (Fbln7-C) inhibits tube formation by human umbilical vein endothelial cells (HUVECs) in vitro. In the present study, we examined the in vivo antiangiogenic activity of recombinant full-length Fbln7 (Fbln7-FL) and Fbln7-C proteins using a rat corneal angiogenesis model. We found that both Fbln7-FL and Fbln7-C inhibited neovascularization. Fbln7-C bound to vascular endothelial growth factor receptor 2 (VEGFR2), inhibiting VEGFR2 and ERK phosphorylation and resulting in reduced HUVEC motility. HUVEC attachment to Fbln7-C occurred through an interaction with integrin α5β1 and regulated changes in cellular morphology. These results suggest that Fbln7-C action may target neovascularization by altering cell/ECM associations. Therefore, Fbln7-C could have potential as a therapeutic agent for diseases associated with angiogenesis.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

