Cell-based immunotherapy approaches for multiple myeloma
- PMID: 30518815
- PMCID: PMC6325139
- DOI: 10.1038/s41416-018-0346-9
Cell-based immunotherapy approaches for multiple myeloma
Abstract
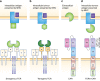
Despite the arrival of novel therapies, multiple myeloma (MM) remains incurable and new treatment options are needed. Chimeric antigen receptor (CAR) T cells are genetically modified T cells that express a CAR directed against specific tumour antigens. CAR T cells are able to kill target tumour cells and may result in long-lasting immune responses in vivo. The rapid development of CAR technologies has led to clinical trials in haematological cancers including MM, and CAR T cells might evolve into a standard treatment in the next few years. Only small patient cohorts with relapsed or refractory disease have so far been investigated, but promising preliminary results with high response rates have been obtained in phase I clinical trials with B cell maturation antigen (BCMA), CD19, CD38 and κ-light-chain CAR T cells. Additional preclinical studies on CD38 and SLAMF7-CAR T cells in MM treatment yielded preclinical results that merit further investigation. Beyond the T cell approach, recent studies have focussed on CAR natural killer (NK) cells in order to increase the reactivity of these effector cells. Finally, to investigate the targeting of intracellular antigens, cellular therapies based on engineered T cell receptors (TCRs) are in development. In this review, we discuss results from preclinical and early-phase clinical trials testing the feasibility and safety of CAR T cell administration in MM, as well as early studies into approaches that utilise CAR NK cell and genetically modified TCRs.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

