Targeting the BRD4/FOXO3a/CDK6 axis sensitizes AKT inhibition in luminal breast cancer
- PMID: 30518851
- PMCID: PMC6281582
- DOI: 10.1038/s41467-018-07258-y
Targeting the BRD4/FOXO3a/CDK6 axis sensitizes AKT inhibition in luminal breast cancer
Abstract
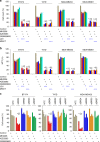
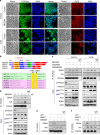
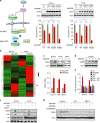
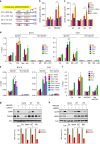
BRD4 assembles transcriptional machinery at gene super-enhancer regions and governs the expression of genes that are critical for cancer progression. However, it remains unclear whether BRD4-mediated gene transcription is required for tumor cells to develop drug resistance. Our data show that prolonged treatment of luminal breast cancer cells with AKT inhibitors induces FOXO3a dephosphorylation, nuclear translocation, and disrupts its association with SirT6, eventually leading to FOXO3a acetylation as well as BRD4 recognition. Acetylated FOXO3a recognizes the BD2 domain of BRD4, recruits the BRD4/RNAPII complex to the CDK6 gene promoter, and induces its transcription. Pharmacological inhibition of either BRD4/FOXO3a association or CDK6 significantly overcomes the resistance of luminal breast cancer cells to AKT inhibitors in vitro and in vivo. Our study reports the involvement of BRD4/FOXO3a/CDK6 axis in AKTi resistance and provides potential therapeutic strategies for treating resistant breast cancer.
Conflict of interest statement
The authors declare no competing interests.
Figures


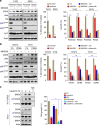
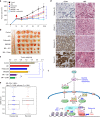
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

