Direct Cardiac Actions of Sodium Glucose Cotransporter 2 Inhibitors Target Pathogenic Mechanisms Underlying Heart Failure in Diabetic Patients
- PMID: 30519189
- PMCID: PMC6259641
- DOI: 10.3389/fphys.2018.01575
Direct Cardiac Actions of Sodium Glucose Cotransporter 2 Inhibitors Target Pathogenic Mechanisms Underlying Heart Failure in Diabetic Patients
Abstract
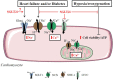
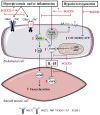
Sodium glucose cotransporter 2 inhibitors (SGLT2i) are the first antidiabetic compounds that effectively reduce heart failure hospitalization and cardiovascular death in type 2 diabetics. Being explicitly designed to inhibit SGLT2 in the kidney, SGLT2i have lately been investigated for their off-target cardiac actions. Here, we review the direct effects of SGLT2i Empagliflozin (Empa), Dapagliflozin (Dapa), and Canagliflozin (Cana) on various cardiac cell types and cardiac function, and how these may contribute to the cardiovascular benefits observed in large clinical trials. SGLT2i impaired the Na+/H+ exchanger 1 (NHE-1), reduced cytosolic [Ca2+] and [Na+] and increased mitochondrial [Ca2+] in healthy cardiomyocytes. Empa, one of the best studied SGLT2i, maintained cell viability and ATP content following hypoxia/reoxygenation in cardiomyocytes and endothelial cells. SGLT2i recovered vasoreactivity of hyperglycemic and TNF-α-stimulated aortic rings and of hyperglycemic endothelial cells. Anti-inflammatory actions of Cana in IL-1β-treated HUVEC and of Dapa in LPS-treated cardiofibroblast were mediated by AMPK activation. In isolated mouse hearts, Empa and Cana, but not Dapa, induced vasodilation. In ischemia-reperfusion studies of the isolated heart, Empa delayed contracture development during ischemia and increased mitochondrial respiration post-ischemia. Direct cardiac effects of SGLT2i target well-known drivers of diabetes and heart failure (elevated cardiac cytosolic [Ca2+] and [Na+], activated NHE-1, elevated inflammation, impaired vasorelaxation, and reduced AMPK activity). These cardiac effects may contribute to the large beneficial clinical effects of these antidiabetic drugs.
Keywords: 2 type diabetes; SGLT2 inhibitors; cardiac fibroblast; cardiomyocyte; endothelial cell; heart failure; isolated heart; smooth muscle cell.
Figures


References
-
- Azcutia V., Abu-Taha M., Romacho T., Vázquez-Bella M., Matesanz N., Luscinskas F. W., et al. (2010). Inflammation determines the pro-adhesive properties of high extracellular D-glucose in human endothelial cells in vitro and rat microvessels in vivo. PLoS One 5:e10091. 10.1371/journal.pone.0010091 - DOI - PMC - PubMed
-
- Baartscheer A., Hardziyenka M., Schumacher C. A., Belterman C. N. W., Van Borren M. M., Verkerk A. O., et al. (2008). Chronic inhibition of the Na+/H+-exchanger causes regression of hypertrophy, heart failure, and ionic and electrophysiological remodelling. Br. J. Pharmacol. 154 1266–1275. 10.1038/bjp.2008.189 - DOI - PMC - PubMed
-
- Baartscheer A., Schumacher C. A., Van Belterman M. M., Borren C. N., Coronel R., Fiolet J. W. (2003b). Increased Na+/H+-exchange activity is the cause of increased [Na+]I and underlies disturbed calcium handling in the rabbit pressure and volume overload heart failure model. Heart Fail. 57 1015–1024. 10.1016/S0008-6363(02)00809-X - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

