Split-face Evaluation of a Multi-ingredient Brightening Foam Versus a Reference Control in Women with Photodamaged Facial Skin
- PMID: 30519376
- PMCID: PMC6239159
Split-face Evaluation of a Multi-ingredient Brightening Foam Versus a Reference Control in Women with Photodamaged Facial Skin
Abstract
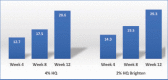
Background: The gold standard for the treatment of hyperpigmentation is hydroquinone (HQ), which has been available as a skin lightener for more than 50 years. Numerous clinical studies have proven its efficacy in various topical formulations. In the United States, HQ is available as a nonprescription product in 2% formulations and as a 4% prescription product. Objective: This study compared the safety and efficacy of a 2% hydroquinone multi-ingredient foam with a standard 4% hydroquinone cream on photodamaged facial skin. Methods: A 12-week, investigator-blinded, randomized trial with a split-face design was conducted in women with moderate photodamaged facial skin. Results: Both products improved the appearance of photodamaged facial skin and were well-tolerated. No statistically significant changes were seen between treatments during the efficacy or tolerability evaluations. Conclusion: Both treatments (2% HQ Brighten and 4% HQ) improved the appearance of photodamaged facial skin and were well-tolerated and results well-perceived by subjects over the 12-week treatment period, compared with baseline grading scores.
Keywords: Arbutin; foam delivery vehicle; hydroquinone; hyperpigmentation; kojic acid; niacinamide; photodamaged facial skin.
Conflict of interest statement
FUNDING:This study was funded by Precision Dermatology. DISCLOSURES:Mr. Gotz reports stock options with MDRejuvena, Inc. The other authors have no conflicts of interest relevant to the content of this article.
Figures





References
-
- Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol. 2001;79(6):547–568. - PubMed
-
- Ortonne JP, Bissett DL. Latest insights into skin hyperpigmentation. J Invest Dermatol Symp Proc. 2008;13(1):10–14. - PubMed
-
- Gilchrest BA. Skin aging and photoaging: an overview. J Am Acad Dermatol. 1989;21(3 Pt 2):610–613. - PubMed
-
- Fisher GJ, Kang S, Varani J et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138(11):1462–1470. - PubMed
-
- Taylor CR, Stern RS, Leyden JJ, Gilchrest BA. Photoaging/photodamage and photoprotection. J Am Acad Dermatol. 1990;22(1):1–15. - PubMed
LinkOut - more resources
Full Text Sources
