Clinical Course of Dual-Chamber Implantable Cardioverter-Defibrillator Recipients followed by Cardiac Remote Monitoring: Insights from the LION Registry
- PMID: 30519574
- PMCID: PMC6241353
- DOI: 10.1155/2018/3120480
Clinical Course of Dual-Chamber Implantable Cardioverter-Defibrillator Recipients followed by Cardiac Remote Monitoring: Insights from the LION Registry
Abstract
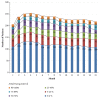
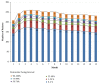
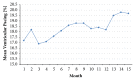
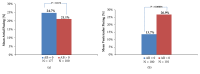
Patients receiving dual-chamber implantable cardioverter-defibrillator (DR-ICD) therapy are at risk of developing atrial arrhythmia because of the increased rate of ventricular pacing and the progression of heart failure. Remote monitoring (RM) may identify the patients at highest risk of adverse events such as atrial arrhythmias. A total of 283 patients with 91,632 remote transmissions during a 15-month follow-up (FU) period enrolled in the LION registry were analysed. The parameters retrieved included the pacing mode, lower rate limit, percentage of atrial (%AP) and ventricular pacing (%VP), and percentage of atrial arrhythmia burden (%AB). In 92.7% of patients, the devices were initially programmed in DDD(R) or DDI(R), with changes of the pacing mode in 19.3% only. The lower rate limit remained stable in 80.4% of patients. At the first transmission, 8.7% of patients suffered from RM-detected atrial arrhythmia, which reached 36% during FU. The %AP was not associated with increased AB (p = 0.67), but the %VP was different in patients developing RM-detected atrial arrhythmia (26.9% vs. 13.7%, p < 0.00001). The %VP increased in 105 patients (significance level of α = 0.05), and 11 patients crossed the border of 50% VP. The LION substudy supports the concept of using RM in a real-world DR-ICD population. Remote monitoring of DR-ICDs allows for the quantification of the course of the pacing parameters and AB. Based on these observations, device parameters can be adjusted and optimized.
Figures
Similar articles
-
Multicenter, prospective, randomized safety and efficacy study of a new atrial-based managed ventricular pacing mode (MVP) in dual chamber ICDs.J Cardiovasc Electrophysiol. 2005 Aug;16(8):811-7. doi: 10.1111/j.1540-8167.2005.40766.x. J Cardiovasc Electrophysiol. 2005. PMID: 16101620 Clinical Trial.
-
Importance of the atrial channel for ventricular arrhythmia therapy in the dual chamber implantable cardioverter defibrillator.J Cardiovasc Electrophysiol. 2000 Dec;11(12):1309-19. doi: 10.1046/j.1540-8167.2000.01309.x. J Cardiovasc Electrophysiol. 2000. PMID: 11196552 Clinical Trial.
-
Impact of COVID-19 on the incidence of cardiac arrhythmias in implantable cardioverter defibrillator recipients followed by remote monitoring.Arch Cardiovasc Dis. 2021 May;114(5):407-414. doi: 10.1016/j.acvd.2021.02.005. Epub 2021 May 24. Arch Cardiovasc Dis. 2021. PMID: 34088625 Free PMC article.
-
Should all implantable cardioverter defibrillators for ventricular arrhythmias be dual-chamber devices?Curr Cardiol Rep. 2001 Nov;3(6):447-50. doi: 10.1007/s11886-001-0065-2. Curr Cardiol Rep. 2001. PMID: 11602074 Review.
-
Remote monitoring: Doomed to let down or an attractive promise?Int J Cardiol Heart Vasc. 2019 May 30;24:100380. doi: 10.1016/j.ijcha.2019.100380. eCollection 2019 Sep. Int J Cardiol Heart Vasc. 2019. PMID: 31193998 Free PMC article. Review.
References
-
- Zipes D. P., Camm A. J., Borggrefe M. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death—executive summary: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. European Heart Journal. 2006;27(17):2099–2140. doi: 10.1093/eurheartj/ehl199. - DOI - PubMed
-
- Tracy C. M., Epstein A. E., Darbar D., et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American college of cardiology foundation/American heart association task force on practice guidelines and the heart rhythm society. Journal of the American College of Cardiology. 2013;61(3):e6–e75. doi: 10.1016/j.jacc.2012.11.007. - DOI - PubMed
-
- Bardy G. H., Lee K. L., Mark D. B., et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. New England Journal of Medicine. 2005;352(3):225–237. - PubMed
-
- Yancy C. W., Jessup M., Bozkurt B., et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/american heart association task force on practice guidelines. Journal of the American College of Cardiology. 2013;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. - DOI - PubMed
-
- Wilkoff B. L., Cook J. R., Epstein A. E., et al. Dual-chamber pacing-or ventricular backup pacing in patients with an implantable defibrillator: The Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. Journal of the American Medical Association. 2002;288(24):3115–3123. doi: 10.1001/jama.288.24.3115. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

