Prospective randomised controlled trial of Algisite™ M, Cuticerin™, and Sorbact® as donor site dressings in paediatric split-thickness skin grafts
- PMID: 30519595
- PMCID: PMC6267093
- DOI: 10.1186/s41038-018-0135-y
Prospective randomised controlled trial of Algisite™ M, Cuticerin™, and Sorbact® as donor site dressings in paediatric split-thickness skin grafts
Abstract
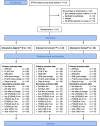
Background: This is a parallel three-arm prospective randomised controlled trial (RCT) comparing Algisite™ M, Cuticerin™, and Sorbact® as donor site dressings in paediatric split-thickness skin grafts (STSG). All three were in current use within the Pegg Leditschke Children's Burn centre (PLCBC), the largest paediatric burns centre in Queensland, Australia. Our objective was to find the best performing dressing, following on from previous trials designed to rationalise dressings for the burn wound itself.
Methods: All children for STSG, with thigh donor sites, were considered for enrolment in the trial. Primary outcome measures were days to re-epithelialisation, and pain. Secondary measures were cost, itch, and scarring at 3 and 6 months. Patients and parents were blinded to group assignment. Blinding of assessors was possible with the dressing in situ, with partial blinding following first dressing change. Blinded photographic assessments of re-epithelialisation were used. Scar assessment was blinded. Covariates for analysis were sex, age, and graft thickness (as measured from a central biopsy).
Results: There were 101 patients randomised to the Algisite™ M (33), Cuticerin™ (32), and Sorbact® (36) arms between April 2015 and July 2016. All were analysed for time to re-epithelialisation. Pain scores were not available for all time points in all patients. There were no significant differences between the three arms regarding pain, or time to re-epithelialisation. There were no significant differences for the secondary outcomes of itch, scarring, or cost. Regression analyses demonstrated faster re-epithelialisation in younger patients and decreased donor site scarring at 3 and 6 months with thinner STSG. There were no adverse effects noted.
Conclusions: There are no data supporting a preference for one trial dressing over the others, in donor site wounds (DSW) in children. Thinner skin grafts lead to less donor site scarring in children. Younger patients have faster donor site wound healing.
Trial registration: Australia and New Zealand Clinical Trials Register (ACTRN12614000380695).Royal Children's Hospital Human Research Ethics Committee (HREC/14/QRCH/36).University of Queensland Medical Research Ethics Committee (#2014000447).
Keywords: Alginate; Algisite™ M; Burns; Cuticerin™; Donor site wound; Paediatric; Sorbact®; Split-thickness skin graft.
Conflict of interest statement
Craig A McBride FRACS is a Senior Staff Specialist Paediatric Surgeon with Children’s Health Queensland. He is a Senior Paediatric Burns Surgeon with the PLCBC. This RCT forms part of a research higher degree. Roy M Kimble MD, FRCS, FRACS is Professor of Paediatric Surgery and Director of Paediatric Surgery at Lady Cilento Children’s Hospital (Children’s Health Services Queensland). He is a Senior Paediatric Burns Surgeon with the PLCBC. Kellie Stockton PhD, BApp Sc (Physio), Post Grad Dip Physio (cardiothoracic) was Clinical Research Manager of the Centre for Children’s Burns and Trauma Research (CCBTR) at the time this study was being conducted. She is now the Director of Physiotherapy for Children’s Health Queensland.This trial was approved by the Royal Children’s Hospital Human Research Ethics Committee (HREC/14/QRCH/36) and the University of Queensland Medical Research Ethics Committee (#2014000447). All patients were entered after signed consent.Not applicable.None of the authors have any financial ties to Abigo Medical AB. None of the authors have received honoraria, travel, or accommodation from the company. None of the authors have undertaken speaking engagements at the request of the company. RMK and CAM have both accepted invitations, with attendant travel and accommodation, from Smith & Nephew to speak at meetings and conferences regarding the treatment of paediatric burns and the use of negative pressure wound therapy in children. RMK has provided medico-legal opinion for Smith & Nephew. Smith & Nephew had no involvement in the conduct of this trial. The authors declare that they have no competing interests.
Figures
Similar articles
-
Three donor site dressings in pediatric split-thickness skin grafts: study protocol for a randomised controlled trial.Trials. 2015 Feb 8;16:43. doi: 10.1186/s13063-015-0557-9. Trials. 2015. PMID: 25887128 Free PMC article. Clinical Trial.
-
The use of modern dressings in managing split-thickness skin graft donor sites: a single-centre randomised controlled trial.J Wound Care. 2017 Jun 2;26(6):281-291. doi: 10.12968/jowc.2017.26.6.281. J Wound Care. 2017. PMID: 28598760 Clinical Trial.
-
Comparison of three different dressings for partial thickness burns in children: study protocol for a randomised controlled trial.Trials. 2013 Nov 25;14:403. doi: 10.1186/1745-6215-14-403. Trials. 2013. PMID: 24274190 Free PMC article. Clinical Trial.
-
Biological versus non-biological dressings in the management of split-thickness skin-graft donor sites: a systematic review and meta-analysis.J Wound Care. 2020 Oct 2;29(10):604-610. doi: 10.12968/jowc.2020.29.10.604. J Wound Care. 2020. PMID: 33052797
-
Polyurethane Versus Calcium Alginate Dressings for Split-Thickness Skin Graft Donor Site: A Systematic Review and Meta-Analysis.Cureus. 2021 Nov 30;13(11):e20027. doi: 10.7759/cureus.20027. eCollection 2021 Nov. Cureus. 2021. PMID: 34987912 Free PMC article. Review.
Cited by
-
Systematic literature review of topical local anaesthesia or analgesia to donor site wounds.Burns Trauma. 2022 Sep 19;10:tkac020. doi: 10.1093/burnst/tkac020. eCollection 2022. Burns Trauma. 2022. PMID: 36133279 Free PMC article.
-
First-Line Interactive Wound Dressing Update: A Comprehensive Review of the Evidence.Front Pharmacol. 2020 Feb 28;11:155. doi: 10.3389/fphar.2020.00155. eCollection 2020. Front Pharmacol. 2020. PMID: 32180720 Free PMC article. Review.
-
Compounds of Marine Origin with Possible Applications as Healing Agents.Mar Drugs. 2024 Dec 26;23(1):5. doi: 10.3390/md23010005. Mar Drugs. 2024. PMID: 39852507 Free PMC article. Review.
-
Randomized clinical trial of negative pressure wound therapy as an adjunctive treatment for small-area thermal burns in children.Br J Surg. 2020 Dec;107(13):1741-1750. doi: 10.1002/bjs.11993. Epub 2020 Sep 14. Br J Surg. 2020. PMID: 32926410 Free PMC article. Clinical Trial.
-
The feasibility of negative pressure wound therapy versus standard dressings in paediatric hand and foot burns protocol: a pilot, single-centre, randomised control trial.Pilot Feasibility Stud. 2023 May 26;9(1):90. doi: 10.1186/s40814-023-01308-z. Pilot Feasibility Stud. 2023. PMID: 37237316 Free PMC article.
References
-
- Geary PM, Tiernan E. Management of split skin graft donor sites–results of a national survey. Clin Plast Surg. 2012;39:77–84. - PubMed
-
- Vermeulen H, Ubbink DT, de Zwart F, Goossens A, de Vos R. Preferences of patients, doctors, and nurses regarding wound dressing characteristics: a conjoint analysis. Wound Repair Regen. 2007;15:302–7. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

