Patients carrying CYP2C8*3 have shorter systemic paclitaxel exposure
- PMID: 30520341
- PMCID: PMC6562943
- DOI: 10.2217/pgs-2018-0162
Patients carrying CYP2C8*3 have shorter systemic paclitaxel exposure
Abstract
Aim: First, evaluate if patients carrying putatively diminished activity CYP2C8 genotype have longer paclitaxel exposure (e.g., time above threshold concentration of 0.05 μM [Tc >0.05]). Second, screen additional pharmacogenes for associations with Tc >0.05. Methods: Pharmacogene panel genotypes were translated into genetic phenotypes for associations with Tc >0.05 (n = 58).
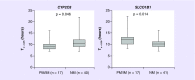
Results: Patients with predicted low-activity CYP2C8 had shorter Tc >0.05 after adjustment for age, body surface area and race (9.65 vs 11.03 hrs, β = 5.47, p = 0.02). This association was attributed to CYP2C8*3 (p = 0.006), not CYP2C8*4 (p = 0.58). Patients with predicted low-activity SLCO1B1 had longer Tc >0.05 (12.12 vs 10.15 hrs, β = 0.85, p = 0.012).
Conclusion: Contrary to previous publications, CYP2C8*3 may confer increased paclitaxel metabolic activity. SLCO1B1 and CYP2C8 genotype may explain some paclitaxel pharmacokinetic variability.
Keywords: paclitaxel; pharmacogenomics.
Conflict of interest statement
This work was supported by the National Center for Advancing Translational Sciences under award number KL2TR000434 and 2UL1TR000433 (DL Hertz) and the National Cancer Institutes of Health under Award Number P30CA046592 (KM Kidwell).
DL Hertz has an informal, unpaid collaborative relationship with Saladax Biomedical Inc., a company that offers CLIA-approved paclitaxel measurement. Saladax was not involved in the design, conduct, analysis, or sponsorship of this trial, and had no contribution to the writing of this manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures
References
-
- Perez EA. Paclitaxel in breast cancer. Oncologist. 1998;3(6):373–389. - PubMed
-
- Mielke S, Sparreboom A, Steinberg SM, et al. Association of paclitaxel pharmacokinetics with the development of peripheral neuropathy in patients with advanced cancer. Clin. Cancer Res. 2005;11(13):4843–4850. - PubMed
-
•• Utilizes their previously collected clinical and paclitaxel pharmacokinetic data to show that patients that developed peripheral neuropathy had greater paclitaxel exposure, including the parameter time above threshold.
-
- Mielke S, Sparreboom A, Behringer D, Mross K. Paclitaxel pharmacokinetics and response to chemotherapy in patients with advanced cancer treated with a weekly regimen. Anticancer Res. 2005;25(6C):4423–4427. - PubMed
-
• Determines that the parameter, time above threshold, was associated with paclitaxel treatment response.
-
- Hertz DL, Kidwell KM, Vangipuram K, et al. Paclitaxel plasma concentration after the first infusion predicts treatment-limiting peripheral neuropathy. Clin. Cancer Res. 2018;24(15):3602–3610. - PMC - PubMed
-
• In the cohort of patients included in the manuscript, increased paclitaxel exposure was predictive of peripheral neuropathy-induced treatment disruption.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
