Investigation of the contribution of an underlying platelet defect in women with unexplained heavy menstrual bleeding
- PMID: 30520671
- PMCID: PMC6406209
- DOI: 10.1080/09537104.2018.1543865
Investigation of the contribution of an underlying platelet defect in women with unexplained heavy menstrual bleeding
Abstract
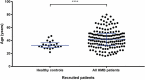
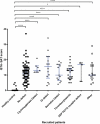
Heavy menstrual bleeding (HMB) is often undiagnosed in women and can cause discomfort and distress. A haemostatic cause for excessive bleeding is often not routinely investigated and can lead to hysterectomy at an early age. A prospective cohort study was carried out to determine whether certain patients with unexplained HMB have an underlying platelet function defect (PFD). The Genotyping and Phenotyping of Platelets (GAPP) study recruited 175 women with HMB and 44 unrelated volunteers from 25 Haemophilia Centres across the UK, and a tertiary gynaecology service. Bleeding history was assessed using the International Society on Thrombosis and Haemostasis Bleeding Assessment Tool (ISTH-BAT). Platelet count, platelet size, haemoglobin and mean corpuscular volume were measured in whole blood using the Sysmex XN-1000 Haematology Analyzer. Platelet function testing using lumiaggregometry and flow cytometry was performed in patients included in this study. A PFD was identified in 47% (82/175) of patients with HMB. Cutaneous bleeding was the most frequent additional bleeding symptom (89% in PFD and 83% with no PFD). Whole blood platelet count was significantly lower (P < 0.0001) between the PFD group and no PFD group. The prevalence of anaemia did not differ between patients and healthy volunteers. Clinical evaluation alone is insufficient to determine presence of an underlying PFD in patients with HMB. Platelet function tests may be considered and clinical guidelines may include them in their algorithms. An appropriate diagnosis and subsequent tailored management of HMB may prevent unnecessary surgery and help manage future haemostatic challenges.
Keywords: Aggregometry; bleeding; heavy menstrual bleeding; platelet function defects; platelet function tests; platelets.
Figures




References
-
- Wyatt KM, Dimmock PW, Walker TJ, O’Brien PM. Determination of total menstrual blood loss.Fertil Steril 2001;76(1):125–131. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
