Paths to Successful Translation of New Therapies for Severe Traumatic Brain Injury in the Golden Age of Traumatic Brain Injury Research: A Pittsburgh Vision
- PMID: 30520681
- PMCID: PMC7698994
- DOI: 10.1089/neu.2018.6203
Paths to Successful Translation of New Therapies for Severe Traumatic Brain Injury in the Golden Age of Traumatic Brain Injury Research: A Pittsburgh Vision
Abstract
New neuroprotective therapies for severe traumatic brain injury (TBI) have not translated from pre-clinical to clinical success. Numerous explanations have been suggested in both the pre-clinical and clinical arenas. Coverage of TBI in the lay press has reinvigorated interest, creating a golden age of TBI research with innovative strategies to circumvent roadblocks. We discuss the need for more robust therapies. We present concepts for traditional and novel approaches to defining therapeutic targets. We review lessons learned from the ongoing work of the pre-clinical drug and biomarker screening consortium Operation Brain Trauma Therapy and suggest ways to further enhance pre-clinical consortia. Biomarkers have emerged that empower choice and assessment of target engagement by candidate therapies. Drug combinations may be needed, and it may require moving beyond conventional drug therapies. Precision medicine may also link the right therapy to the right patient, including new approaches to TBI classification beyond the Glasgow Coma Scale or anatomical phenotyping-incorporating new genetic and physiologic approaches. Therapeutic breakthroughs may also come from alternative approaches in clinical investigation (comparative effectiveness, adaptive trial design, use of the electronic medical record, and big data). The full continuum of care must also be represented in translational studies, given the important clinical role of pre-hospital events, extracerebral insults in the intensive care unit, and rehabilitation. TBI research from concussion to coma can cross-pollinate and further advancement of new therapies. Misconceptions can stifle/misdirect TBI research and deserve special attention. Finally, we synthesize an approach to deliver therapeutic breakthroughs in this golden age of TBI research.
Keywords: clinical trial design; combination therapy; consortium; neuroprotection; pharmacodynamics/response biomarker; phenotyping; quantitative systems pharmacology; rehabilitation; target engagement; traumatic brain injury (TBI).
Conflict of interest statement
Dr. Ruchira Jha is a Consultant for Biogen. Dr. D. Lansing Taylor is the co-founder and chairman of SpIntellx Inc., co-founder and advisor of Cernostics, Inc., and advisor of Von Baer Wolff, Inc. The remaining authors declare no competing financial interests exist.
Figures

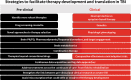


References
-
- Centers for Disease Control and Prevention. Traumatic Brain Injury–Related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2007 and 2013. 2017. https://www.cdc.gov/mmwr/volumes/66/ss/ss6609a1.htm Accessed September15, 2018 - PMC - PubMed
-
- http://apps.webofknowledge.com/WOS_GeneralSearch_input.do?product=WOS&se.... Accessed April16, 2018
-
- Kochanek P.M., Bramlett H., Dietrich W.D., Dixon C.E., Hayes R.L., Povlishock J., Tortella F.C., and Wang K.K. (2011). A novel multicenter preclinical drug screening and biomarker consortium for experimental traumatic brain injury: Operation Brain Trauma Therapy. J. Trauma 71, 1 Suppl., S15–S24 - PubMed
-
- Ji J., Kline A.E., Amoscato A., Arias A.S., Sparvero L.J., Tyurin V.A., Tyurina Y.Y., Fink B., Manole M.D., Puccio A.M., Okonkwo D.O., Cheng J.P., Alexander H., Clark R.S., Kochanek P.M., Wipf P., Kagan V.E., and Bayır H. (2012). Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nat. Neurosci. 15, 1407–1415 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

