Hereditary spastic paraplegia: gain-of-function mechanisms revealed by new transgenic mouse
- PMID: 30520996
- PMCID: PMC6423423
- DOI: 10.1093/hmg/ddy419
Hereditary spastic paraplegia: gain-of-function mechanisms revealed by new transgenic mouse
Abstract
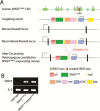
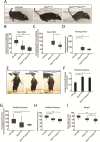
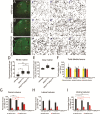
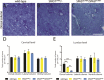
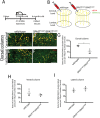
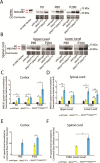
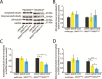
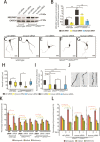
Mutations of the SPAST gene, which encodes the microtubule-severing protein spastin, are the most common cause of hereditary spastic paraplegia (HSP). Haploinsufficiency is the prevalent opinion as to the mechanism of the disease, but gain-of-function toxicity of the mutant proteins is another possibility. Here, we report a new transgenic mouse (termed SPASTC448Y mouse) that is not haploinsufficient but expresses human spastin bearing the HSP pathogenic C448Y mutation. Expression of the mutant spastin was documented from fetus to adult, but gait defects reminiscent of HSP (not observed in spastin knockout mice) were adult onset, as is typical of human patients. Results of histological and tracer studies on the mouse are consistent with progressive dying back of corticospinal axons, which is characteristic of the disease. The C448Y-mutated spastin alters microtubule stability in a manner that is opposite to the expectations of haploinsufficiency. Neurons cultured from the mouse display deficits in organelle transport typical of axonal degenerative diseases, and these deficits were worsened by depletion of endogenous mouse spastin. These results on the SPASTC448Y mouse are consistent with a gain-of-function mechanism underlying HSP, with spastin haploinsufficiency exacerbating the toxicity of the mutant spastin proteins. These findings reveal the need for a different therapeutic approach than indicated by haploinsufficiency alone.
© The Author(s) 2018. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Hazan J., Fonknechten N., Mavel D., Paternotte C., Samson D., Artiguenave F., Davoine C.S., Cruaud C., Durr A., Wincker P. et al. (1999) Spastin, a new AAA protein, is altered in the most frequent form of autosomal dominant spastic paraplegia. Nat. Genet., 23, 296–303. - PubMed
-
- Fonknechten N., Mavel D., Byrne P., Davoine C.S., Cruaud C., Bonsch D., Samson D., Coutinho P., Hutchinson M., McMonagle P. et al. (2000) Spectrum of SPG4 mutations in autosomal dominant spastic paraplegia. Hum. Mol. Genet., 9, 637–644. - PubMed
-
- Burger J., Fonknechten N., Hoeltzenbein M., Neumann L., Bratanoff E., Hazan J. and Reis A. (2000) Hereditary spastic paraplegia caused by mutations in the SPG4 gene. Eur. J. Hum. Genet., 8, 771–776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

