Differences in Peripheral and Tissue Immune Cell Populations Following Haematopoietic Stem Cell Transplantation in Crohn's Disease Patients
- PMID: 30521002
- PMCID: PMC6486491
- DOI: 10.1093/ecco-jcc/jjy203
Differences in Peripheral and Tissue Immune Cell Populations Following Haematopoietic Stem Cell Transplantation in Crohn's Disease Patients
Abstract
Background and aims: Recent studies have shown the efficacy of autologous haematopoietic stem cell transplantation [HSCT] in severely refractory Crohn's disease [CD] patients. HSCT is thought to eliminate auto-reactive cells; however, no specific studies of immune reconstitution in CD patients are available.
Methods: We followed a group of CD patients [n = 18] receiving autologous HSCT, with 50% of them achieving endoscopic drug-free remission. To elucidate the mechanisms driving efficacy, we monitored changes after HSCT in blood and intestine immune-cell composition. CD patients [n = 22] receiving anti-tumour necrosis factor [TNF]-α were included for comparison.
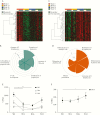
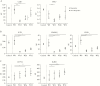
Results: Severe immune ablation followed by HSCT induced dramatic changes in both peripheral blood T and B cells in all patients regardless of the efficacy of the treatment. Endoscopic remission at week 52 following HSCT was associated with significant intestinal transcriptional changes. A comparison of the remission signature with that of anti-TNFα identified both common and unique genes in the HSCT-induced response. Based on deconvolution analysis of intestinal biopsy transcriptome data, we show that response to HSCT, but not to anti-TNFα, is associated with an expansion of naïve B-cells, as seen in blood, and a decrease in the memory resting T-cell content. As expected, endoscopic remission, in response to both HSCT and anti-TNFα, led to a significant reduction in intestinal neutrophil and M1 macrophage content.
Conclusions: Peripheral blood immune remodelling after HSCT does not predict efficacy. In contrast, a profound intestinal T-cell depletion that is maintained long after transplant is associated with mucosal healing following HSCT, but not anti-TNFα.
Keywords: Crohn’s disease; anti-TNFα; autologous haematopoietic stem cell transplantation.
© European Crohn’s and Colitis Organisation (ECCO) 2018.
Figures






References
-
- Hawkey CJ, Allez M, Clark MM, et al. .. Autologous hematopoetic stem cell transplantation for refractory Crohn disease: a randomized clinical trial. JAMA 2015;314:2524–34. - PubMed
-
- Jauregui-Amezaga A, Rovira M, Marín P, et al. .. Improving safety of autologous haematopoietic stem cell transplantation in patients with Crohn’s disease. Gut 2016;65:1456–62. - PubMed
-
- Saccardi R, Kozak T, Bocelli-Tyndall C, et al. .; Autoimmune Diseases Working Party of EBMT Autologous stem cell transplantation for progressive multiple sclerosis: update of the European Group for Blood and Marrow Transplantation autoimmune diseases working party database. Mult Scler 2006;12:814–23. - PubMed
-
- Sormani MP, Muraro PA, Schiavetti I, et al. .. Autologous hematopoietic stem cell transplantation in multiple sclerosis: a meta-analysis. Neurology 2017;88:2115–22. - PubMed
-
- Alchi B, Jayne D, Labopin M, et al. .; EBMT Autoimmune Disease Working Party members Autologous haematopoietic stem cell transplantation for systemic lupus erythematosus: data from the European Group for Blood and Marrow Transplantation registry. Lupus 2013;22:245–53. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

