Development and validation of a haematuria cancer risk score to identify patients at risk of harbouring cancer
- PMID: 30521125
- PMCID: PMC6446724
- DOI: 10.1111/joim.12868
Development and validation of a haematuria cancer risk score to identify patients at risk of harbouring cancer
Abstract
Background: A lack of consensus exists amongst national guidelines regarding who should be investigated for haematuria. Type of haematuria and age-specific thresholds are frequently used to guide referral for the investigation of haematuria.
Objectives: To develop and externally validate the haematuria cancer risk score (HCRS) to improve patient selection for the investigation of haematuria.
Methods: Development cohort comprise of 3539 prospectively recruited patients recruited at 40 UK hospitals (DETECT 1; ClinicalTrials.gov: NCT02676180) and validation cohort comprise of 656 Swiss patients. All patients were aged >18 years and referred to hospital for the evaluation of visible and nonvisible haematuria. Sensitivity and specificity of the HCRS in the validation cohort were derived from a cut-off identified from the discovery cohort.
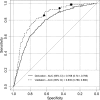
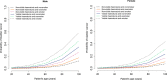
Results: Patient age, gender, type of haematuria and smoking history were used to develop the HCRS. HCRS validation achieves good discrimination (AUC 0.835; 95% CI: 0.789-0.880) and calibration (calibration slope = 1.215) with no significant overfitting (P = 0.151). The HCRS detected 11.4% (n = 8) more cancers which would be missed by UK National Institute for Health and Clinical Excellence guidelines. The American Urological Association guidelines would identify all cancers with a specificity of 12.6% compared to 30.5% achieved by the HCRS. All patients with upper tract cancers would have been identified.
Conclusion: The HCRS offers good discriminatory accuracy which is superior to existing guidelines. The simplicity of the model would facilitate adoption and improve patient and physician decision-making.
Keywords: bladder cancer; detection; haematuria; nomogram; predict; urinary tract cancer.
© 2018 The Authors. Journal of Internal Medicine published by John Wiley & Sons Ltd on behalf of Association for Publication of The Journal of Internal Medicine.
Conflict of interest statement
None reported.
Figures
References
-
- Linder BJ, Bass EJ, Mostafid H, Boorjian SA. Guideline of guidelines: asymptomatic microscopic haematuria. BJU Int 2017; 16: 14016. - PubMed
-
- National Institute for Health and Care Excellence . Suspected cancer: recognition and referral. 2015. - PubMed
-
- Davis R, Jones JS, Barocas DA et al Diagnosis, evaluation and follow‐up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol 2012; 188: 2473–81. - PubMed
-
- Tan WS, Feber A, Sarpong R et al Who Should Be Investigated for Haematuria? Results of a Contemporary Prospective Observational Study of 3556 Patients. Eur Urol 2018; 74: 10–14. - PubMed
-
- Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008; 26: 1364–70. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

