Interleukin-6 inhibition of hERG underlies risk for acquired long QT in cardiac and systemic inflammation
- PMID: 30521586
- PMCID: PMC6283635
- DOI: 10.1371/journal.pone.0208321
Interleukin-6 inhibition of hERG underlies risk for acquired long QT in cardiac and systemic inflammation
Abstract
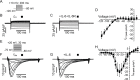
Increased proinflammatory interleukin-6 (IL-6) levels are associated with acquired long QT-syndrome (LQTS) in patients with systemic inflammation, leading to higher risks for life-threatening polymorphic ventricular tachycardia such as Torsades de Pointes. However, the functional and molecular mechanisms of this association are not known. In most cases of acquired LQTS, the target ion channel is the human ether-á-go-go-related gene (hERG) encoding the rapid component of the delayed rectifier K current, IKr, which plays a critical role in cardiac repolarization. Here, we tested the hypothesis that IL-6 may cause QT prolongation by suppressing IKr. Electrophysiological and biochemical assays were used to assess the impact of IL-6 on the functional expression of IKr in HEK293 cells and adult guinea-pig ventricular myocytes (AGPVM). In HEK293 cells, IL-6 alone or in combination with the soluble IL-6 receptor (IL-6R), produced a significant depression of IKr peak and tail current densities. Block of IL-6R or Janus kinase (JAK) reversed the inhibitory effects of IL-6 on IKr. In AGPVM, IL-6 prolonged action potential duration (APD) which was further prolonged in the presence of IL-6R. Similar to heterologous cells, IL-6 reduced endogenous guinea pig ERG channel mRNA and protein expression. The data are first to demonstrate that IL-6 inhibition of IKr and the resulting prolongation of APD is mediated via IL-6R and JAK pathway activation and forms the basis for the observed clinical QT interval prolongation. These novel findings may guide the development of targeted anti-arrhythmic therapeutic interventions in patients with LQTS and inflammatory disorders.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


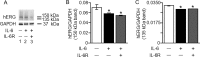




References
-
- Yang S, Zheng R, Hu S, Ma Y, Choudhry MA, Messina JL, et al. Mechanism of cardiac depression after trauma-hemorrhage: increased cardiomyocyte IL-6 and effect of sex steroids on IL-6 regulation and cardiac function. American journal of physiology Heart and circulatory physiology. 2004;287(5):H2183–91. Epub 2004/10/12. 10.1152/ajpheart.00624.2003 . - DOI - PubMed
-
- Fontes JA, Rose NR, Cihakova D. The varying faces of IL-6: From cardiac protection to cardiac failure. Cytokine. 2015;74(1):62–8. Epub 2015/02/05. 10.1016/j.cyto.2014.12.024 ; PubMed Central PMCID: PMCPmc4677779. - DOI - PMC - PubMed
-
- Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6: from basic science to medicine. Arthritis Res. 2002;4 Suppl 3:S233–42. Epub 2002/07/12. 10.1186/ar565 ; PubMed Central PMCID: PMCPMC3240141. - DOI - PMC - PubMed
-
- Kurdi M, Booz GW. Can the protective actions of JAK-STAT in the heart be exploited therapeutically? Parsing the regulation of interleukin-6-type cytokine signaling. J Cardiovasc Pharmacol. 2007;50(2):126–41. Epub 2007/08/19. 10.1097/FJC.0b013e318068dd49 . - DOI - PubMed
-
- Lazzerini PE, Laghi-Pasini F, Bertolozzi I, Morozzi G, Lorenzini S, Simpatico A, et al. Systemic inflammation as a novel QT-prolonging risk factor in patients with torsades de pointes. Heart (British Cardiac Society). 2017. Epub 2017/05/12. 10.1136/heartjnl-2016-311079 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

