Inhaled steroids with and without regular salmeterol for asthma: serious adverse events
- PMID: 30521673
- PMCID: PMC6524619
- DOI: 10.1002/14651858.CD006922.pub4
Inhaled steroids with and without regular salmeterol for asthma: serious adverse events
Abstract
Background: Epidemiological evidence has suggested a link between use of beta₂-agonists and increased asthma mortality. Much debate has surrounded possible causal links for this association, and whether regular (daily) long-acting beta₂-agonists (LABAs) are safe, particularly when used in combination with inhaled corticosteroids (ICSs). This is an update of a Cochrane Review that now includes data from two large trials including 11,679 adults and 6208 children; both were mandated by the US Food and Drug Administration (FDA). OBJECTIVES: To assess risks of mortality and non-fatal serious adverse events (SAEs) in trials that randomised participants with chronic asthma to regular salmeterol and ICS versus the same dose of ICS.
Search methods: We identified randomised trials using the Cochrane Airways Group Specialised Register of trials. We checked websites of clinical trials registers for unpublished trial data. We also checked FDA submissions in relation to salmeterol. The date of the most recent search was 10 October 2018.
Selection criteria: We included parallel-design randomised trials involving adults, children, or both with asthma of any severity who were randomised to treatment with regular salmeterol and ICS (in separate or combined inhalers) versus the same dose of ICS of at least 12 weeks in duration.
Data collection and analysis: We conducted the review according to standard procedures expected by Cochrane. We obtained unpublished data on mortality and SAEs from the sponsors, from ClinicalTrials.gov, and from FDA submissions. We assessed our confidence in the evidence according to current GRADE recommendations.
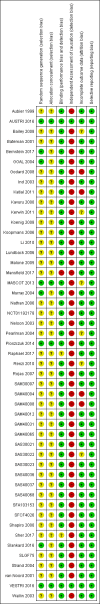
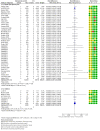
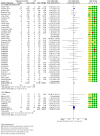
Main results: We have included in this review 41 studies (27,951 participants) in adults and adolescents, along with eight studies (8453 participants) in children. We judged that the overall risk of bias was low for all-cause events, and we obtained data on SAEs from all study authors. All except 542 adults (and none of the children) were given salmeterol and fluticasone in the same (combination) inhaler.DeathsEleven of a total of 14,233 adults taking regular salmeterol and ICS died, as did 13 of 13,718 taking regular ICS at the same dose. The pooled Peto odds ratio (OR) was 0.80 (95% confidence interval (CI) 0.36 to 1.78; participants = 27,951; studies = 41; I² = 0%; moderate-certainty evidence). In other words, for every 1000 adults treated for 25 weeks, one death occurred among those on ICS alone, and the corresponding risk among those taking salmeterol and ICS was also one death (95% CI 0 to 2 deaths).No children died, and no adults or children died of asthma, so we remain uncertain about mortality in children and about asthma mortality in any age group.Non-fatal serious adverse eventsA total of 332 adults receiving regular salmeterol with ICS experienced a non-fatal SAE of any cause, compared to 282 adults receiving regular ICS. The pooled Peto OR was 1.14 (95% CI 0.97 to 1.33; participants = 27,951; studies = 41; I² = 0%; moderate-certainty evidence). For every 1000 adults treated for 25 weeks, 21 adults on ICS alone had an SAE, and the corresponding risk for those on salmeterol and ICS was 23 adults (95% CI 20 to 27).Sixty-five of 4229 children given regular salmeterol with ICS suffered an SAE of any cause, compared to 62 of 4224 children given regular ICS. The pooled Peto OR was 1.04 (95% CI 0.73 to 1.48; participants = 8453; studies = 8; I² = 0%; moderate-certainty evidence). For every 1000 children treated for 23 weeks, 15 children on ICS alone had an SAE, and the corresponding risk for those on salmeterol and ICS was 15 children (95% CI 11 to 22).Asthma-related serious adverse eventsEighty and 67 adults in each group, respectively, experienced an asthma-related non-fatal SAE. The pooled Peto OR was 1.15 (95% CI 0.83 to 1.59; participants = 27,951; studies = 41; I² = 0%; low-certainty evidence). For every 1000 adults treated for 25 weeks, five receiving ICS alone had an asthma-related SAE, and the corresponding risk among those on salmeterol and ICS was six adults (95% CI 4 to 8).Twenty-nine children taking salmeterol and ICS and 23 children taking ICS alone reported asthma-related events. The pooled Peto OR was 1.25 (95% CI 0.72 to 2.16; participants = 8453; studies = 8; I² = 0%; moderate-certainty evidence). For every 1000 children treated for 23 weeks, five receiving an ICS alone had an asthma-related SAE, and the corresponding risk among those receiving salmeterol and ICS was seven children (95% CI 4 to 12).
Authors' conclusions: We did not find a difference in the risk of death or serious adverse events in either adults or children. However, trial authors reported no asthma deaths among 27,951 adults or 8453 children randomised to regular salmeterol and ICS or ICS alone over an average of six months. Therefore, the risk of dying from asthma on either treatment was very low, but we remain uncertain about whether the risk of dying from asthma is altered by adding salmeterol to ICS.Inclusion of new trials has increased the precision of the estimates for non-fatal SAEs of any cause. We can now say that the worst-case estimate is that at least 152 adults and 139 children must be treated with combination salmeterol and ICS for six months for one additional person to be admitted to the hospital (compared to treatment with ICS alone). These possible risks still have to be weighed against the benefits experienced by people who take combination treatment.However more than 90% of prescribed treatment was taken in the new trials, so the effects observed may be different from those seen with salmeterol in combination with ICS in daily practice.
Conflict of interest statement
Previous versions
Roman Jaeschke received honoraria for lectures from Boehringer Ingelheim (2006; USD4000) and GlaxoSmithKline (2007; EUR2000), as well as travel support from Boehringer Ingelheim and GlaxoSmithKline (2006 and 2007; up to USD1000). This occurred more than five years before publication of the review.
Mattthew Cates and Toby Lasserson: none known.
Figures





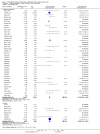
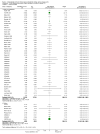
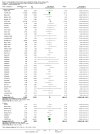
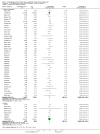
Update of
-
Regular treatment with salmeterol and inhaled steroids for chronic asthma: serious adverse events.Cochrane Database Syst Rev. 2013 Mar 28;(3):CD006922. doi: 10.1002/14651858.CD006922.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2018 Dec 03;12:CD006922. doi: 10.1002/14651858.CD006922.pub4. PMID: 23543548 Updated.
References
References to studies included in this review
-
- Aubier M, Pieters WR, Schlösser NJ, Steinmetz KO. Salmeterol/fluticasone propionate (50/500 microg) in combination in a Diskus inhaler (Seretide) is effective and safe in the treatment of steroid‐dependent asthma. Respiratory Medicine 1999;93:876‐84. - PubMed
- SFCB3019. A multicentre randomized, double‐blind, double‐dummy, parallel‐group comparison of the salmeterol/fluticasone propionate combination product (50/500mcg strength) BD via one DISKUS/Accuhaler inhaler with salmeterol 50mcg BD via one DISKUS/Accuhaler and fluticasone propionate 500mcg BD via another .... www.gsk‐clinicalstudyregister.com/study/SFCB3019#rs (first received 28 September 2008).
-
- NCT01475721. SAS115359, a safety and efficacy study of inhaled fluticasone propionate/salmeterol combination versus inhaled fluticasone propionate in the treatment of adolescent and adult subjects with asthma. clinicaltrials.gov/ct2/show/NCT01475721 (first received 21 November 2011).
- Stempel DA, Raphiou I, Kral K, Yeakey A, Buaron K, Emmett A, et al. Austri, a large randomized study in adolescents and adults with asthma, assessing the safety and efficacy of salmeterol in combination with fluticasone propionate compared to fluticasone propionate alone. Journal of Allergy and Clinical Immunology2016; Vol. 137, issue 2:Suppl 1: AB389.
- Stempel DA, Raphiou IH, Kral KM, Yeakey AM, Emmett MS, Prazma CM, et al. Serious asthma events with fluticasone plus salmeterol versus fluticasone alone. New England Journal of Medicine 2016;374(19):1822‐30. [DOI: 10.1056/NEJMoa1511049] - DOI - PubMed
-
- Bailey W, Castro M, Matz J, White M, Dransfield M, Yancey S, et al. Asthma exacerbations in African Americans treated for 1 year with combination fluticasone propionate and salmeterol or fluticasone propionate alone. Current Medical Research and Opinion2008; Vol. 24, issue 6:1669‐82. [NCT00102765] - PubMed
- SFA103153. A multicenter, randomized, double‐blind, parallel group, 52‐week comparison of asthma control and measures of airway inflammation in subjects of African descent receiving fluticasone propionate/salmeterol100/50mcg DISKUS™ bid or fluticasone propionate 100mcg DISKUS™ bid alone. www.gsk‐clinicalstudyregister.com/study/SFA103153 (first received 26 October 2012). [SFA103153]
-
- Bateman ED, Silins V, Bogolubov M. Clinical equivalence of salmeterol/fluticasone propionate in combination (50/100 micrograms twice daily) when administered via a chlorofluorocarbon‐free metered dose inhaler or dry powder inhaler to patients with mild‐to‐moderate asthma. Respiratory Medicine 2001;95:136‐46. - PubMed
- SFCB3022. A multicentre, randomised, double‐blind, double‐dummy, parallel‐group, three‐month comparison of the salmeterol/fluticasone propionate combination product (2x25/50mcg strength) bd via the pressurised metered dose inhaler with salmeterol/fluticasone propionate combination product (1x50/100mcg strength) bd via the Diskus/Accuhaler™ inhaler and with fluticasone propionate (2x 50mcg strength) alone bd via the pressurised metered dose inhaler in adolescents and adults with reversible airways obstruction. www.gsk‐clinicalstudyregister.com/study/SFCB3022#rs (first received 28 September 2008).
-
- Bernstein D, Andersen L, Forth R, Jacques L, Yates L. Once‐daily fluticasone furoate/vilanterol versus twice‐daily fluticasone propionate/salmeterol in patients with asthma well controlled on ICS/LABA. Journal of Asthma 2017 April 13 [Epub ahead of print]. [DOI: 10.1080/02770903.2017.1386214] - DOI
- Bernstein D, Forth R, Jacques L, Andersen L, Yates L. Comparison of once daily fluticasone furoate/vilanterol with twice daily fluticasone propionate/ salmeterol in patients with controlled asthma. Chest 2017;152(4 Suppl 1):A186. [DOI: 10.1016/j.chest.2017.08.217] - DOI
References to studies excluded from this review
-
- Adinoff AD, Schwartz HJ, Rickard KA, Yancey SW, Swearingen BE. Salmeterol compared with current therapies in chronic asthma.[erratum appears in Journal of Family Practice 1999 Jan;48(1):67]. Journal of Family Practice 1998;47(4):278‐84. - PubMed
-
- Adolfsson LE, Lundgren M, Tilling B, Jern S, Tyren C, Godwood A, et al. Short‐term safety and tolerability of double‐dose salmeterol/fluticasone propionate in adult asthmatic patients. Clinical Drug Investigation 2005;25(4):231‐41. - PubMed
-
- Bateman ED, Britton M, Carrillo J, Almeida J, Wixon C. Salmeterol/fluticasone combination inhaler. A new, effective and well tolerated treatment for asthma. Clinical Drug Investigation 1998;16(3):193‐201. - PubMed
-
- Bateman ED, Jacques L, Goldfrad C, Atienza T, Mihaescu T, Duggan M. Asthma control can be maintained when fluticasone propionate/salmeterol in a single inhaler is stepped down. Journal of Allergy and Clinical Immunology 2006;117(3):563‐70. - PubMed
-
- Bateman ED, Kornmann O, Schmidt P, Pivovarova A, Engel M, Fabbri LM. Tiotropium is noninferior to salmeterol in maintaining improved lung function in B16‐Arg/Arg patients with asthma. Journal of Allergy and Clinical Immunology 2011;128(2):315‐22. - PubMed
References to ongoing studies
-
- NCT02980133. Study of fluticasone propionate multidose dry powder inhaler compared with fluticasone propionate/salmeterol multidose dry powder inhaler. clinicaltrials.gov/show/NCT02980133 (first received 2 December 2016). - PubMed
Additional references
-
- Anderson GP. Current issues with beta(2)‐adrenoceptor agonists ‐ pharmacology and molecular and cellular mechanisms. Clinical Reviews in Allergy and Immunology 2006;31(2‐3):119‐30. - PubMed
-
- Barnes PJ. Beta‐adrenoceptors on smooth‐muscle, nerves and inflammatory cells. Life Sciences 1993;52(26):2101‐9. - PubMed
-
- Barnes PJ. Beta‐adrenergic receptors and their regulation. American Journal of Respiratory and Critical Care Medicine 1995;152(3):838‐60. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

