Pharmacological interventions for acute hepatitis C infection
- PMID: 30521693
- PMCID: PMC6517308
- DOI: 10.1002/14651858.CD011644.pub3
Pharmacological interventions for acute hepatitis C infection
Abstract
Background: Hepatitis C virus (HCV) is a single-stranded RNA (ribonucleic acid) virus that has the potential to cause inflammation of the liver. The traditional definition of acute HCV infection is the first six months following infection with the virus. Another commonly used definition of acute HCV infection is the absence of HCV antibody and subsequent seroconversion (presence of HCV antibody in a person who was previously negative for HCV antibody). Approximately 40% to 95% of people with acute HCV infection develop chronic HCV infection, that is, have persistent HCV RNA in their blood. In 2010, an estimated 160 million people worldwide (2% to 3% of the world's population) had chronic HCV infection. The optimal pharmacological treatment of acute HCV remains controversial. Chronic HCV infection can damage the liver.
Objectives: To assess the comparative benefits and harms of different pharmacological interventions in the treatment of acute HCV infection through a network meta-analysis and to generate rankings of the available pharmacological treatments according to their safety and efficacy. However, it was not possible to assess whether the potential effect modifiers were similar across different comparisons. Therefore, we did not perform the network meta-analysis and instead we assessed the comparative benefits and harms of different interventions versus each other or versus no intervention using standard Cochrane methodology.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, Science Citation Index Expanded, World Health Organization International Clinical Trials Registry Platform, and randomised controlled trials registers to April 2016 to identify randomised clinical trials on pharmacological interventions for acute HCV infection.
Selection criteria: We included only randomised clinical trials (irrespective of language, blinding, or publication status) in participants with acute HCV infection. We excluded trials which included previously liver transplanted participants and those with other coexisting viral diseases. We considered any of the various pharmacological interventions compared with placebo or each other.
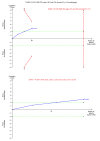
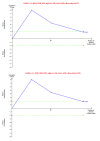
Data collection and analysis: We used standard methodological procedures expected by Cochrane. We calculated the odds ratio (OR) and rate ratio with 95% confidence intervals (CI) using both fixed-effect and random-effects models based on the available-participant analysis with Review Manager 5. We assessed risk of bias according to Cochrane, controlled risk of random errors with Trial Sequential Analysis, and assessed the quality of the evidence using GRADE.
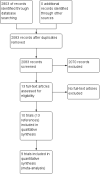
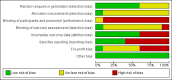
Main results: We identified 10 randomised clinical trials with 488 randomised participants that met our inclusion criteria. All the trials were at high risk of bias in one or more domains. Overall, the evidence for all the outcomes was very low quality evidence. Nine trials (467 participants) provided information for one or more outcomes. Three trials (99 participants) compared interferon-alpha versus no intervention. Three trials (90 participants) compared interferon-beta versus no intervention. One trial (21 participants) compared pegylated interferon-alpha versus no intervention, but it did not provide any data for analysis. One trial (41 participants) compared MTH-68/B vaccine versus no intervention. Two trials (237 participants) compared pegylated interferon-alpha versus pegylated interferon-alpha plus ribavirin. None of the trials compared direct-acting antivirals versus placebo or other interventions. The mean or median follow-up period in the trials ranged from six to 36 months.There was no short-term mortality (less than one year) in any group in any trial except for one trial where one participant died in the pegylated interferon-alpha plus ribavirin group (1/95: 1.1%). In the trials that reported follow-up beyond one year, there were no further deaths. The number of serious adverse events was higher with pegylated interferon-alpha plus ribavirin than with pegylated interferon-alpha (rate ratio 2.74, 95% CI 1.40 to 5.33; participants = 237; trials = 2; I2 = 0%). The proportion of people with any adverse events was higher with interferon-alpha and interferon-beta compared with no intervention (OR 203.00, 95% CI 9.01 to 4574.81; participants = 33; trials = 1 and OR 27.88, 95% CI 1.48 to 526.12; participants = 40; trials = 1). None of the trials reported health-related quality of life, liver transplantation, decompensated liver disease, cirrhosis, or hepatocellular carcinoma. The proportion of people with chronic HCV infection as indicated by the lack of sustained virological response was lower in the interferon-alpha group versus no intervention (OR 0.27, 95% CI 0.09 to 0.76; participants = 99; trials = 3; I2 = 0%). The differences between the groups were imprecise or not estimable (because neither group had any events) for all the remaining comparisons.Four of the 10 trials (40%) received financial or other assistance from pharmaceutical companies who would benefit from the findings of the research; the source of funding was not available in five trials (50%), and one trial (10%) was funded by a hospital.
Authors' conclusions: Very low quality evidence suggests that interferon-alpha may decrease the incidence of chronic HCV infection as measured by sustained virological response. However, the clinical impact such as improvement in health-related quality of life, reduction in cirrhosis, decompensated liver disease, and liver transplantation has not been reported. It is also not clear whether this finding is applicable in the current clinical setting dominated by the use of pegylated interferons and direct-acting antivirals, although we found no evidence to support that pegylated interferons or ribavirin or both are effective in people with acute HCV infection. We could find no randomised trials comparing direct-acting antivirals with placebo or other interventions for acute HCV infection. There is significant uncertainty in the benefits and harms of the interventions, and high-quality randomised clinical trials are required.
Conflict of interest statement
This report is independent research funded by the National Institute for Health Research (NIHR Cochrane Programme Grants, 13/89/03 ‐ evidence‐based diagnosis and management of upper digestive, hepato‐biliary, and pancreatic disorders). The views expressed in this publication are those of the review authors and not necessarily those of the National Health Service (NHS), the NIHR, or the Department of Health.
MK: no financial disclosures. EB: no financial disclosures. DT: Astellas funded Douglas Thorburn for his attendance at the International Liver Transplantation Society meeting in 2014. Douglas Thorburn has also received GBP 25,000 from Boston Scientific to fund a clinical research fellow in 2013. There are no other financial disclosures to report. BD: no financial disclosures. ET: no financial disclosures. KG: no financial disclosures.
Figures


Update of
-
Pharmacological interventions for acute hepatitis C infection: an attempted network meta-analysis.Cochrane Database Syst Rev. 2017 Mar 13;3(3):CD011644. doi: 10.1002/14651858.CD011644.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2018 Dec 03;12:CD011644. doi: 10.1002/14651858.CD011644.pub3. PMID: 28285495 Free PMC article. Updated.
References
References to studies included in this review
Calleri 1998 {published data only}
-
- Calleri G, Colombatto P, Gozzelino M, Chieppa F, Romano P, Delmastro B, et al. Natural beta interferon in acute type‐C hepatitis patients: a randomized controlled trial. Italian Journal of Gastroenterology and Hepatology 1998;30(2):181‐4. - PubMed
Csatary 1998 {published data only}
-
- Csatary LK, Telegdy L, Gergely P, Bodey B, Bakacs T. Preliminary report of a controlled trial of MTH‐68/B virus vaccine treatment in acute B and C hepatitis: a phase II study. Anticancer Research 1998;18(2B):1279‐82. - PubMed
Deterding 2013 {published data only}
-
- Deterding K, Gruner N, Buggisch P, Wiegand J, Gale PR, Spengler U, et al. Delayed versus immediate treatment for patients with acute hepatitis C: a randomised controlled non‐inferiority trial. Lancet Infectious Diseases 2013;13(6):497‐506. - PubMed
Genesca 1993 {published data only}
-
- Genesca J. Interferon alfa in acute posttransfusion hepatitis C: a randomized, controlled trial. Gastroenterology 1992;103(5):1702‐3. - PubMed
Hwang 1994 {published data only}
-
- Hwang SJ, Lee SD, Chan CY, Lu RH, Lo KJ. A randomized controlled trial of recombinant interferon alpha‐2b in the treatment of Chinese patients with acute post‐transfusion hepatitis C. Journal of Hepatology 1994;21(5):831‐6. - PubMed
-
- Hwang SJ, Lee SD, Lee YH, Wu JC, Chan CY, Huang YS, et al. A randomized controlled clinical trial of recombinant interferon alpha‐2b in the treatment of acute post‐transfusion hepatitis C: a preliminary report. Journal of Gastroenterology and Hepatology 1993;8:S92‐8.
Lampertico 1994 {published data only}
-
- Lampertico P, Rumi M, Romeo R, Craxi A, Soffredini R, Biassoni D, et al. A multicenter randomized controlled trial of recombinant interferon‐alpha 2b in patients with acute transfusion‐associated hepatitis C. Hepatology 1994;19(1):19‐22. - PubMed
-
- Lampertico P, Rumi MG, De FF, Romeo R, Soffredini R, Biassoni D, et al. Treatment of acute post‐transfusional non‐a, non‐b hepatitis with recombinant interferon alpha‐2b. A multicenter randomized controlled trial. Argomenti Gastroenterologia Clinica 1994;7(3):111‐5.
Omata 1991 {published data only}
-
- Omata M, Yokosuka O, Takano S, Kato N, Hosoda K, Imazeki F, et al. Resolution of acute hepatitis C after therapy with natural beta interferon. Lancet 1991;338(8772):914‐5. - PubMed
Omata 1994 {published data only}
-
- Omata M, Takano S. A randomized, controlled trial of interferon‐beta treatment for acute hepatitis C. Proceedings of the international symposium on viral hepatitis and liver Disease. 1st Edition. Tokyo: Springer‐Verlag, 1994:601‐3.
Santantonio 2014 {published data only}
-
- Santantonio T, Fasano M, Sagnelli E, Tundo P, Babudieri S, Fabris P, et al. Acute hepatitis C: a 24‐week course of pegylated interferon alpha‐2b versus a 12‐week course of pegylated interferon alpha‐2b alone or with ribavirin. Hepatology 2014;59(6):2101‐9. - PubMed
Wang 2005 {published data only}
-
- Wang C, Cook L, Krows M, Ng K, Hough E, Thiede H. Randomized trial of pegylated interferon for the treatment of acute HCV in Seattle injection drug users. Hepatology 2005;42(4 Suppl 1):673a.
Additional references
AASLD 2014
-
- American Association for the Study of Liver Diseases, Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C, 2014. www.hcvguidelines.org/fullreport (accessed 12 October 2014).
Anonymous 1981
Bailon 2001
-
- Bailon P, Palleroni A, Schaffer CA, Spence CL, Fung WJ, Porter JE, et al. Rational design of a potent, long‐lasting form of interferon: a 40 kDa branched polyethylene glycol‐conjugated interferon alpha‐2a for the treatment of hepatitis C. Bioconjugate Chemistry 2001;12(2):195‐202. - PubMed
Beinhardt 2012
-
- Beinhardt S, Aberle JH, Strasser M, Dulic‐Lakovic E, Maieron A, Kreil A, et al. Serum level of IP‐10 increases predictive value of IL28B polymorphisms for spontaneous clearance of acute HCV infection. Gastroenterology 2012;142(1):78‐85. - PubMed
Beltrami 2000
Bunchorntavakul 2015
Chaimani 2012
-
- Chaimani A, Salanti G. Using network meta‐analysis to evaluate the existence of small‐study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161‐76. - PubMed
Chaimani 2013
Chan 2013
Coilly 2014
-
- Coilly A, Roche B, Dumortier J, Leroy V, Botta‐Fridlund D, Radenne S, et al. Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation, a multicenter experience. Journal of Hepatology 2014;60(1):78‐86. - PubMed
D'Amico 2006
-
- D'Amico G, Garcia‐Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. Journal of Hepatology 2006;44(1):217‐31. - PubMed
Del Re 2013
Demets 1987
-
- Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Dias 2010
-
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Statistics in Medicine 2010;29(7‐8):932‐44. - PubMed
Dias 2012a
-
- Dias S, Sutton AJ, Welton NJ, Ades AE. NICE DSU Technical Support Document 3: heterogeneity: subgroups, meta‐regression, bias and bias‐adjustment, September 2011 (last updated April 2012). www.nicedsu.org.uk/TSD3%20Heterogeneity.final%20report.08.05.12.pdf (accessed 27 March 2014).
Dias 2012b
-
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 1: introduction to evidence synthesis for decision making, April 2011 (last updated April 2012). www.nicedsu.org.uk/TSD1%20Introduction.final.08.05.12.pdf (accessed 27 March 2014).
Dias 2014a
-
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: a generalised linear modelling framework for pairwise and network meta‐analysis of randomised controlled trials, August 2011 (last updated April 2014). www.nicedsu.org.uk/TSD2%20General%20meta%20analysis%20corrected%2015Apri... (accessed 8 October 2014). - PubMed
Dias 2014b
-
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU Technical Support Document 4: inconsistency in networks of evidence based on randomised controlled trials, May 2011 (last updated April 2014). www.nicedsu.org.uk/TSD4%20Inconsistency.final.15April2014.pdf (accessed 8 October 2014). - PubMed
EASL 2014
-
- European Association for Study of Liver. EASL clinical practice guidelines: management of hepatitis C virus infection. Journal of Hepatology 2014;60(2):392‐420. - PubMed
Egger 1997
El Khoury 2012
-
- Khoury AC, Wallace C, Klimack WK, Razavi H. Economic burden of hepatitis C‐associated diseases: Europe, Asia Pacific, and the Americas. Journal of Medical Economics 2012;15(5):887‐96. - PubMed
EuroQol 2014
-
- EuroQol. About EQ‐5D, 2014. www.euroqol.org/about‐eq‐5d.html (accessed 8 October 2014).
Feld 2005
-
- Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature 2005;436(7053):967‐72. - PubMed
Fierer 2014
Furusyo 2013
-
- Furusyo N, Ogawa E, Nakamuta M, Kajiwara E, Nomura H, Dohmen K, et al. Telaprevir can be successfully and safely used to treat older patients with genotype 1b chronic hepatitis C. Journal of Hepatology 2013;59(2):205‐12. - PubMed
Global Burden of Hepatitis C Working Group 2004
-
- Global Burden of Hepatitis C Working Group. Global burden of disease (GBD) for hepatitis C. Journal of Clinical Pharmacology 2004;44(1):20‐9. - PubMed
Gluud 2014
-
- Gluud C, Koretz R, Gurusamy K. Hepatitis C: a new direction, but an old story?. Lancet (London, England) 2014; Vol. 383, issue 9935:2122‐3. [PUBMED: 24953469] - PubMed
Gluud 2015
-
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2015, Issue 1. Art. No.: LIVER.
Grebely 2011
-
- Grebely J, Matthews GV, Dore GJ. Treatment of acute HCV infection. Nature Reviews. Gastroenterology & Hepatology 2011;8(5):265‐74. - PubMed
Grebely 2014
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. - PubMed
Hajarizadeh 2012
-
- Hajarizadeh B, Grebely J, Dore GJ. Case definitions for acute hepatitis C virus infection: a systematic review. Journal of Hepatology 2012;57(6):1349‐60. - PubMed
Hezode 2013
-
- Hezode C, Fontaine H, Dorival C, Larrey D, Zoulim F, Canva V, et al. Triple therapy in treatment‐experienced patients with HCV‐cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20‐CUPIC) ‐ NCT01514890. Journal of Hepatology 2013;59(3):434‐41. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2012
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, Pennsylvania: Barnett International/PAREXEL, 1997.
Jafari 2010
-
- Jafari S, Copes R, Baharlou S, Etminan M, Buxton J. Tattooing and the risk of transmission of hepatitis C: a systematic review and meta‐analysis. International Journal of Infectious Diseases 2010;14(11):e928‐40. - PubMed
Jakobsen 2014
Kamal 2008
-
- Kamal SM. Acute hepatitis C: a systematic review. American Journal of Gastroenterology 2008;103(5):1283‐97. - PubMed
Kenny‐Walsh 1999
-
- Kenny‐Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti‐D immune globulin. Irish Hepatology Research Group. New England Journal of Medicine 1999;340(16):1228‐33. - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Kleinman 2009
-
- Kleinman SH, Lelie N, Busch MP. Infectivity of human immunodeficiency virus‐1, hepatitis C virus, and hepatitis B virus and risk of transmission by transfusion. Transfusion 2009;49(11):2454‐89. - PubMed
Koretz 2015
-
- Koretz RL, Lin KW, Ioannidis JP, Lenzer J. Is widespread screening for hepatitis C justified?. BMJ (Clinical Research Ed.) 2015;350:g7809. - PubMed
Lavanchy 2011
-
- Lavanchy D. Evolving epidemiology of hepatitis C virus. Clinical Microbiology and Infection 2011;17(2):107‐15. - PubMed
Lehmann 2004
-
- Lehmann M, Meyer MF, Monazahian M, Tillmann HL, Manns MP, Wedemeyer H. High rate of spontaneous clearance of acute hepatitis C virus genotype 3 infection. Journal of Medical Virology 2004;73(3):387‐91. - PubMed
Lewis 2012
-
- Lewis H, Cunningham M, Foster G. Second generation direct antivirals and the way to interferon‐free regimens in chronic HCV. Best Practice & Research Clinical Gastroenterology 2012;26(4):471‐85. - PubMed
Loomba 2011
Lu 2006
-
- Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. Journal of the American Statistical Association 2006;101(474):447‐59.
Lundh 2017
Macaskill 2001
-
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. - PubMed
Maheshwari 2008
-
- Maheshwari A, Ray S, Thuluvath PJ. Acute hepatitis C. Lancet 2008;372(9635):321‐32. - PubMed
Martindale 2011
-
- Sweetman S, editor. Martindale: the complete drug reference (online version), 37th edition, 2011. www.pharmpress.com/product/MC_MART/martindale‐the‐complete‐drug‐reference (accessed 23 September 2014).
Mills 2012
-
- Mills EJ, Ioannidis JP, Thorlund K, Schünemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta‐analysis. JAMA 2012;308(12):1246‐53. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. - PubMed
Myers 2001
NCBI 2014a
-
- NCBI. Hepatitis C, 2014. www.ncbi.nlm.nih.gov/mesh/68006526 (accessed 11 October 2014).
NCBI 2014b
-
- NCBI. Interferons, 2014. www.ncbi.nlm.nih.gov/mesh/68007372 (accessed 11 October 2014).
Newell 1992
-
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. - PubMed
OpenBUGS 3.2.3 [Computer program]
-
- Members of OpenBUGS Project Management Group. OpenBUGS. Version 3.2.3. Members of OpenBUGS Project Management Group, 2014.
Perry 2012
-
- Perry CM. Telaprevir: a review of its use in the management of genotype 1 chronic hepatitis C. Drugs 2012;72(5):619‐41. - PubMed
Poordad 2014
-
- Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, et al. ABT‐450/r‐ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. New England Journal of Medicine 2014;370(21):1973‐82. - PubMed
Poynard 1997
-
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997;349(9055):825‐32. - PubMed
Puhan 2014
-
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello‐Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ (Clinical Research Ed.) 2014;349:g5630. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodger 2000
-
- Rodger AJ, Roberts S, Lanigan A, Bowden S, Brown T, Crofts N. Assessment of long‐term outcomes of community‐acquired hepatitis C infection in a cohort with sera stored from 1971 to 1975. Hepatology 2000;32(3):582‐7. - PubMed
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Salanti 2011
-
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. - PubMed
Salanti 2012
-
- Salanti G. Indirect and mixed‐treatment comparison, network, or multiple‐treatments meta‐analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80‐97. - PubMed
Sangiovanni 2006
-
- Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, et al. The natural history of compensated cirrhosis due to hepatitis C virus: a 17‐year cohort study of 214 patients. Hepatology 2006;43(6):1303‐10. - PubMed
Savović 2012a
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. - PubMed
Savović 2012b
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Schulz 2010
Seeff 2001
-
- Seeff LB, Hollinger FB, Alter HJ, Wright EC, Cain CM, Buskell ZJ, et al. Long‐term mortality and morbidity of transfusion‐associated non‐A, non‐B, and type C hepatitis: a National Heart, Lung, and Blood Institute collaborative study. Hepatology 2001;33(2):455‐63. - PubMed
Seeff 2009
Severini 1993
-
- Severini TA. Bayesian interval estimates which are also confidence intervals. Journal of the Royal Statistical Society. Series B (Methodological) 1993;55(2):533‐40.
Singal 2013
-
- Singal AK, Hmoud BS, Guturu P, Kuo YF. Outcome after liver transplantation for cirrhosis due to alcohol and hepatitis C: comparison to alcoholic cirrhosis and hepatitis C cirrhosis. Journal of Clinical Gastroenterology 2013;47(8):727‐33. - PubMed
Smith 2014
Stata/SE 14.2 [Computer program]
-
- StataCorp LP. Stata/SE 14.2 for Windows[64‐bit x86‐64]. Version 14. College Station: StataCorp LP, 2017.
Syriopoulou 2005
-
- Syriopoulou V, Nikolopoulou G, Daikos GL, Theodoridou M, Pavlopoulou I, Nicolaidou P, et al. Mother to child transmission of hepatitis C virus: rate of infection and risk factors. Scandinavian Journal of Infectious Diseases 2005;37(5):350‐3. - PubMed
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 30 November 2016).
Thorlund 2012
Tohme 2010
-
- Tohme RA, Holmberg SD. Is sexual contact a major mode of hepatitis C virus transmission?. Hepatology 2010;52(4):1497‐505. - PubMed
TSA 2011 [Computer program]
-
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
Turner 2012
Uemura 2012
-
- Uemura T, Ramprasad V, Hollenbeak CS, Bezinover D, Kadry Z. Liver transplantation for hepatitis C from donation after cardiac death donors: an analysis of OPTN/UNOS data. American Journal of Transplantation 2012;12(4):984‐91. - PubMed
van Valkenhoef 2012
-
- Valkenhoef G, Lu G, Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta‐analysis. Research Synthesis Methods 2012;3(4):285‐99. - PubMed
Ware 2014
-
- Ware JE. SF‐36® health survey update, 2014. www.sf‐36.org/tools/sf36.shtml (accessed on 8 October 2014).
Wawrzynowicz‐Syczewska 2004
-
- Wawrzynowicz‐Syczewska M, Kubicka J, Lewandowski Z, Boron‐Kaczmarska A, Radkowski M. Natural history of acute symptomatic hepatitis type C. Infection 2004;32(3):138‐43. - PubMed
Welsch 2012
-
- Welsch C, Jesudian A, Zeuzem S, Jacobson I. New direct‐acting antiviral agents for the treatment of hepatitis C virus infection and perspectives. Gut 2012;61(Suppl 1):i36‐46. - PubMed
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wiese 2005
-
- Wiese M, Grungreiff K, Guthoff W, Lafrenz M, Oesen U, Porst H. Outcome in a hepatitis C (genotype 1b) single source outbreak in Germany ‐ a 25‐year multicenter study. Journal of Hepatology 2005;43(4):590‐8. - PubMed
Wood 2008
Xia 2008
-
- Xia X, Luo J, Bai J, Yu R. Epidemiology of hepatitis C virus infection among injection drug users in China: systematic review and meta‐analysis. Public Health 2008;122(10):990‐1003. - PubMed
Yang 2014
-
- Yang LS, Shan LL, Saxena A, Morris DL. Liver transplantation: a systematic review of long‐term quality of life. Liver International 2014;34(9):1298‐313. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

