Dual combination therapy versus long-acting bronchodilators alone for chronic obstructive pulmonary disease (COPD): a systematic review and network meta-analysis
- PMID: 30521694
- PMCID: PMC6517098
- DOI: 10.1002/14651858.CD012620.pub2
Dual combination therapy versus long-acting bronchodilators alone for chronic obstructive pulmonary disease (COPD): a systematic review and network meta-analysis
Abstract
Background: Long-acting bronchodilators such as long-acting β-agonist (LABA), long-acting muscarinic antagonist (LAMA), and LABA/inhaled corticosteroid (ICS) combinations have been used in people with moderate to severe chronic obstructive pulmonary disease (COPD) to control symptoms such as dyspnoea and cough, and prevent exacerbations. A number of LABA/LAMA combinations are now available for clinical use in COPD. However, it is not clear which group of above mentioned inhalers is most effective or if any specific formulation works better than the others within the same group or class.
Objectives: To compare the efficacy and safety of available formulations from four different groups of inhalers (i.e. LABA/LAMA combination, LABA/ICS combination, LAMA and LABA) in people with moderate to severe COPD. The review will update previous systematic reviews on dual combination inhalers and long-acting bronchodilators to answer the questions described above using the strength of a network meta-analysis (NMA).
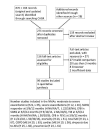
Search methods: We identified studies from the Cochrane Airways Specialised Register, which contains several databases. We also conducted a search of ClinicalTrials.gov and manufacturers' websites. The most recent searches were conducted on 6 April 2018.
Selection criteria: We included randomised controlled trials (RCTs) that recruited people aged 35 years or older with a diagnosis of COPD and a baseline forced expiratory volume in one second (FEV1) of less than 80% of predicted. We included studies of at least 12 weeks' duration including at least two active comparators from one of the four inhaler groups.
Data collection and analysis: We conducted NMAs using a Bayesian Markov chain Monte Carlo method. We considered a study as high risk if recruited participants had at least one COPD exacerbation within the 12 months before study entry and as low risk otherwise. Primary outcomes were COPD exacerbations (moderate to severe and severe), and secondary outcomes included symptom and quality-of-life scores, safety outcomes, and lung function. We collected data only for active comparators and did not consider placebo was not considered. We assumed a class/group effect when a fixed-class model fitted well. Otherwise we used a random-class model to assess intraclass/group differences. We supplemented the NMAs with pairwise meta-analyses.
Main results: We included a total of 101,311 participants from 99 studies (26 studies with 32,265 participants in the high-risk population and 73 studies with 69,046 participants in the low-risk population) in our systematic review. The median duration of studies was 52 weeks in the high-risk population and 26 weeks in the low-risk population (range 12 to 156 for both populations). We considered the quality of included studies generally to be good.The NMAs suggested that the LABA/LAMA combination was the highest ranked treatment group to reduce COPD exacerbations followed by LAMA in the both populations.There is evidence that the LABA/LAMA combination decreases moderate to severe exacerbations compared to LABA/ICS combination, LAMA, and LABA in the high-risk population (network hazard ratios (HRs) 0.86 (95% credible interval (CrI) 0.76 to 0.99), 0.87 (95% CrI 0.78 to 0.99), and 0.70 (95% CrI 0.61 to 0.8) respectively), and that LAMA decreases moderate to severe exacerbations compared to LABA in the high- and low-risk populations (network HR 0.80 (95% CrI 0.71 to 0.88) and 0.87 (95% CrI 0.78 to 0.97), respectively). There is evidence that the LABA/LAMA combination reduces severe exacerbations compared to LABA/ICS combination and LABA in the high-risk population (network HR 0.78 (95% CrI 0.64 to 0.93) and 0.64 (95% CrI 0.51 to 0.81), respectively).There was a general trend towards a greater improvement in symptom and quality-of-life scores with the combination therapies compared to monotherapies, and the combination therapies were generally ranked higher than monotherapies.The LABA/ICS combination was the lowest ranked in pneumonia serious adverse events (SAEs) in both populations. There is evidence that the LABA/ICS combination increases the odds of pneumonia compared to LAMA/LABA combination, LAMA and LABA (network ORs: 1.69 (95% CrI 1.20 to 2.44), 1.78 (95% CrI 1.33 to 2.39), and 1.50 (95% CrI 1.17 to 1.92) in the high-risk population and network or pairwise OR: 2.33 (95% CI 1.03 to 5.26), 2.02 (95% CrI 1.16 to 3.72), and 1.93 (95% CrI 1.29 to 3.22) in the low-risk population respectively). There were significant overlaps in the rank statistics in the other safety outcomes including mortality, total, COPD, and cardiac SAEs, and dropouts due to adverse events.None of the differences in lung function met a minimal clinically important difference criterion except for LABA/LAMA combination versus LABA in the high-risk population (network mean difference 0.13 L (95% CrI 0.10 to 0.15). The results of pairwise meta-analyses generally agreed with those of the NMAs. There is no evidence to suggest intraclass/group differences except for lung function at 12 months in the high-risk population.
Authors' conclusions: The LABA/LAMA combination was the highest ranked treatment group to reduce COPD exacerbations although there was some uncertainty in the results. LAMA containing inhalers may have an advantage over those without a LAMA for preventing COPD exacerbations based on the rank statistics. Combination therapies appear more effective than monotherapies for improving symptom and quality-of-life scores. ICS-containing inhalers are associated with an increased risk of pneumonia.Our most comprehensive review including intraclass/group comparisons, free combination therapies, 99 studies, and 20 outcomes for each high- and low-risk population summarises the current literature and could help with updating existing COPD guidelines.
Conflict of interest statement
Figures







Update of
References
References to studies included in this review
Aaron 2007 {published and unpublished data}
-
- Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone–salmeterol for treatment of chronic obstructive pulmonary disease. Annals of Internal Medicine 2007;146(8):545‐55. [PUBMED: 17310045] - PubMed
Agusti 2014 {published and unpublished data}
-
- Agustí A, Teresa L, Backer W, Zvarich MT, Locantore N, Barnes N, et al. A comparison of the efficacy and safety of once‐daily fluticasone furoate/vilanterol with twice‐daily fluticasone propionate/salmeterol in moderate to very severe COPD. European Respiratory Journal 2014;43(3):763‐72. [PUBMED: 24114969 ] - PubMed
-
- GSK113107. A 12‐week study to evaluate the 24 hour pulmonary function of fluticasone furoate (FF)/vilanterol inhalation powder (FF/VI inhalation powder) once daily compared with salmeterol/fluticasone propionate (FP) inhalation powder twice daily in subjects with chronic obstructive pulmonary disease (COPD). www.gsk‐clinicalstudyregister.com/files2/gsk‐113107‐clinical‐study‐report‐redact... (first received 9 February 2011).
Anzueto 2009 {published and unpublished data}
-
- Anzueto A, Ferguson GT, Feldman G, Chinsky K, Seibert A, Emmett A, et al. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD 2009;6(5):320‐9. [PUBMED: 19863361] - PubMed
-
- GSK100250. A randomized, double‐blind, parallel group, 52‐week study to compare the effect of fluticasone propionate/salmeterol Diskus combination product 250/50mcg bid with salmeterol diskus 50mcg bid on the annual rate of moderate/severe exacerbations in subjects with chronic obstructive pulmonary disease. www.gsk‐clinicalstudyregister.com/files2/gsk‐sco100250‐clinical‐study‐report‐red... (first received 27 December 2004).
Asai 2013 {published and unpublished data}
-
- Asai K, Minakata Y, Hirata K, Fukuchi Y, Kitawaki T, Ikeda K, et al. QVA149 once‐daily is safe and well tolerated and improves lung function and health status in Japanese patients with COPD: the ARISE study. European Respiratory Society 23rd Annual Congress; 2013 September 7‐11; Barcelona. 2013; Vol. A2223.
Bateman 2013 {published and unpublished data}
BI 205.137 2001 {unpublished data only}
-
- BI 205.137. A multiple dose comparison of tiotropium inhalation capsules, salmeterol inhalation aerosol and placebo in a six‐month, double‐blind, double‐dummy, safety and efficacy study in patients with chronic obstructive pulmonary disease (COPD). trials.boehringer‐ingelheim.com/public/trial_results_documents/205/205.137_U01‐1231‐02.pdf (first received 21 February 2001).
Bogdan 2011 {published and unpublished data}
Briggs 2005 {published and unpublished data}
-
- Boehringer Ingelheim International. A multiple dose comparison of tiotropium inhalation capsules and salmeterol inhalation aerosol in a 12 week, randomized, double‐blind, double‐dummy, parallel group study in patients with chronic obstructive pulmonary disease (COPD). trials.boehringer‐ingelheim.com/public/trial_results_documents/205/205.264_CO.pdf 03 FEB 2004. [ClinicalTrials.gov: NCT00274560]
-
- Briggs DD Jr, Covelli H, Lapidus R, Bhattycharya S, Kesten S, Cassino C. Improved daytime spirometric efficacy of tiotropium compared with salmeterol in patients with COPD. Pulmonary Pharmacology and Therapeutics 2005;18(6):397‐404. [PUBMED: 16179215] - PubMed
Brusasco 2003 {published and unpublished data}
-
- Donohue JF, Menjoge S, Kesten S. Tolerance to bronchodilating effects of salmeterol in COPD. Respiratory Medicine 2003;97(9):1014‐20. [PUBMED: 14509555] - PubMed
-
- Donohue JF, Noord JA, Bateman ED, Langley SJ, Lee A, Witek TJ Jr, et al. A 6‐month, placebo‐controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 2002;122(1):47‐55. [PUBMED: 12114338] - PubMed
Buhl 2011 {published and unpublished data}
-
- Buhl R, Dunn LJ, Disdier C, Lassen C, Amos C, Henley M. Blinded 12‐week comparison of once‐daily indacaterol and tiotropium in COPD. European Respiratory Journal 2011;38(4):797‐803. [PUBMED: 21622587 ] - PubMed
Buhl 2015a {published and unpublished data}
Buhl 2015a&b {published and unpublished data}
Buhl 2015b {published and unpublished data}
Buhl 2015c {published and unpublished data}
-
- Buhl R, Gessner C, Schuermann W, Foerster K, Sieder C, Hiltl S, et al. Efficacy and safety of once‐daily QVA149 compared with the free combination of once‐daily tiotropium plus twice‐daily formoterol in patients with moderate‐to‐severe COPD (QUANTIFY): a randomised, non‐inferiority study. Thorax 2015;70(4):311‐9. [PUBMED: 25677679 ] - PMC - PubMed
Calverley 2003 {published and unpublished data}
-
- Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. European Respiratory Journal 2003;22(6):912‐9. [PUBMED: 14680078] - PubMed
Calverley 2003 TRISTAN {published and unpublished data}
-
- Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003;361(9356):449‐56. [PUBMED: 12583942] - PubMed
-
- GSK FCB3024 (Phase III). A multicentre, randomised, double‐blind, parallel group study to compare the efficacy and safety of the salmeterol/fluticasone combination product (50/500mg strength) twice daily with salmeterol 50mg twice daily alone and fluticasone propionate 500mg twice daily alone, all delivered via the Diskus/Accuhaler inhaler, in the treatment of patients with chronic obstructive pulmonary disease. www.gsk‐clinicalstudyregister.com/files2/sfcb3024‐clinical‐study‐report‐redact‐v... (first received 20 August 1998).
Calverley 2007 {published and unpublished data}
-
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. New England Journal of Medicine 2007;356(8):775‐89. [PUBMED: 17314337 ] - PubMed
-
- GSKSCO30003. A multicentre, randomised, double‐blind, parallel group, placebo‐controlled study to investigate the long‐term effects of salmeterol/fluticasone propionate (Seretide) 50/500mcg BD, salmeterol 50mcg BD and fluticasone propionate 500mcg BD, all delivered via the Diskus tm/Accuhaler tm inhaler, on mortality and morbidity of subjects with chronic obstructive pulmonary disease (COPD) over 3 years of treatment. www.gsk‐clinicalstudyregister.com/files2/gsk‐sco30003‐clinical‐study‐report‐reda... (first received 7 September 2000).
Calverley 2010 {published and unpublished data}
-
- Calverley PM, Kuna P, Monsó E, Costantini M, Petruzzelli S, Sergio F, et al. Beclomethasone/formoterol in the management of COPD: a randomised controlled trial. Respiratory Medicine 2010;104(12):1858‐68. [PUBMED: 20965712 ] - PubMed
Cazzola 2007 {published and unpublished data}
-
- Cazzola M, Andò F, Santus P, Ruggeri P, Marco F, Sanduzzi A, et al. A pilot study to assess the effects of combining fluticasone propionate/salmeterol and tiotropium on the airflow obstruction of patients with severe‐to‐very severe COPD. Pulmonary Pharmacology & Therapeutics 2007;20(5):556‐61. [PUBMED: 16914336] - PubMed
Chapman 2014 {published and unpublished data}
-
- Chapman KR, Beeh KM, Beier J, Bateman ED, D'Urzo A, Nutbrown R. A blinded evaluation of the efficacy and safety of glycopyrronium, a once‐daily long‐acting muscarinic antagonist, versus tiotropium, in patients with COPD: the GLOW5 study. BMC Pulmonary Medicine 2014;17(14):4. [PUBMED: 24438744 ] - PMC - PubMed
COMBINE 2017 {unpublished data only}
-
- NCT02055352. 24‐week study to evaluate efficacy and safety of the combination budesonide /indacaterol vs fluticasone /salmeterol in patients with COPD (COMBINE). clinicaltrials.gov/ct2/show/NCT02055352 (first received 5 February 2014).
COSMOS‐J 2016 {unpublished data only}
-
- NCT01762800. Evaluating the control of COPD symptoms in patients treated with tiotropium bromide 18mcg once daily alone, ADOAIR 50/250mcg twice daily alone or ADOAIR 50/250mcg plus tiotropium bromide 18mcg. clinicaltrials.gov/ct2/show/NCT01762800 (first received 8 January 2013).
Covelli 2016 {published and unpublished data}
-
- GSK 115805. A 12‐week study to evaluate the 24‐hour pulmonary function profile of fluticasone furoate/vilanterol (FF/VI) inhalation powder 100/25mcg once‐daily via a novel dry powder inhaler compared with tiotropium bromide inhalation powder 18mcg delivered once‐daily via the HandiHaler in subjects with chronic obstructive pulmonary disease (COPD) who have or are at risk for co‐morbid cardiovascular disease. gsk‐clinicalstudyregister.com/files2/gsk‐115805‐clinical‐study‐report‐redact... (first received 2 April 2012).
D'Urzo 2014 {published and unpublished data}
-
- European Medicines Agency. Assessment report ‐ Duaklir Genuair (aclidinium bromide/ formoterol fumarate dihydrate). www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r... (accessed prior to 15 August 2018).
-
- The Federal Joint Committee (G‐BA) in Germany. Resolution by the Federal Joint Committee on an amendment to the Pharmaceutical Directive (AM‐RL): Appendix XII – Resolutions on the benefit assessment of pharmaceuticals with new active ingredients, in accordance with the German Social Code, Book Five (SGB V), section 35a aclidinium bromide/formoterol. www.english.g‐ba.de/downloads/91‐1028‐156/Aclidinium%20bromide_formoterol_en.pdf (accessed prior to 15 August 2018).
D'Urzo 2017 {published and unpublished data}
-
- D'Urzo A, Rennard S, Kerwin E, Donohue JF, Lei A, Molins E. A randomised double‐blind, placebo‐controlled, long‐term extension study of the efficacy, safety and tolerability of fixed‐dose combinations of aclidinium/formoterol or monotherapy in the treatment of chronic obstructive pulmonary disease. Respiratory Medicine 2017;125:39‐48. [PUBMED: 28340861 ] - PubMed
Dahl 2010 {published and unpublished data}
-
- Dahl R, Chung KF, Buhl R, Magnussen H, Nonikov V, Jack D, et al. Efficacy of a new once‐daily long‐acting inhaled beta2‐agonist indacaterol versus twice‐daily formoterol in COPD. Thorax 2010;65(6):473‐9. [PUBMED: 20522841] - PubMed
Decramer 2013 {published and unpublished data}
-
- Decramer ML, Chapman KR, Dahl R, Frith P, Devouassoux G, Fritscher C, et al. Once‐daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel‐group study. Lancet Respiratory Medicine 2013;1(7):524‐33. [PUBMED: 24461613] - PubMed
Decramer 2014a {published and unpublished data}
-
- Decramer M, Anzueto A, Kerwin E, Kaelin T, Richard N, Crater G, et al. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respiratory Medicine 2014;2(6):472‐86. [PUBMED: 24835833] - PubMed
-
- GSk113360. A multicenter trial comparing the efficacy and safety of GSK573719/GW642444 with GW642444 and with tiotropium over 24 weeks in subjects with COPD. www.gsk‐clinicalstudyregister.com/files2/gsk‐113360‐clinical‐study‐report‐redact... (first received 21 March 2011).
Decramer 2014b {published and unpublished data}
-
- Decramer M, Anzueto A, Kerwin E, Kaelin T, Richard N, Crater G, et al. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respiratory Medicine 2014;2(6):472‐86. [PUBMED: 24835833] - PubMed
-
- GSK113374. A multi‐center trial comparing the efficacy and safety of GSK573719/GW642444 with GSK573719 and with tiotropium over 24 weeks in subjects with COPD. www.gsk‐clinicalstudyregister.com/files2/gsk‐113374‐clinical‐study‐report‐redact... (first received 21 March 2011).
Donohue 2010 {published and unpublished data}
-
- Barnes PJ, Pocock SJ, Magnussen H, Iqbal A, Kramer B, Higgins M, et al. Integrating indacaterol dose selection in a clinical study in COPD using an adaptive seamless design. Pulmonary Pharmacology & Therapeutics 2010;3:165‐71. [ClinicalTrials.gov: NCT00463567; PUBMED: 20080201] - PubMed
-
- Donohue JF, Fogarty C, Lötvall J, Mahler DA, Worth H, Yorgancioglu A, et al. Once‐daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. American Journal of Respiratory and Critical Care Medicine 2010;182(2):155‐62. [PUBMED: 20463178 ] - PubMed
Donohue 2013 {published and unpublished data}
-
- Donohue JF, Maleki‐Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A. Efficacy and safety of once‐daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respiratory Medicine 2013;107(10):1538‐46. [PUBMED: 23830094 ] - PubMed
-
- GSK113374. A 24‐week, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of GSK573719/GW642444 inhalation powder and the individual components delivered once‐daily via a novel dry powder inhaler in subjects with chronic obstructive pulmonary disease. www.gsk‐clinicalstudyregister.com/files2/gsk‐113373‐clinical‐study‐report‐redact... (first received 30 March 2011).
Donohue 2015a {published and unpublished data}
-
- Donohue JF, Worsley S, Zhu CQ, Hardaker L, Church A. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate‐to‐severe COPD and infrequent exacerbations. Respiratory Medicine 2015;109(7):870‐81. [PUBMED: 26006754 ] - PubMed
-
- GSK DB2114930. DB2114930: a randomized, multi‐center, double‐blind, double‐dummy, parallel group study to evaluate the efficacy and safety of umeclidinium/vilanterol compared with fluticasone propionate/salmeterol over 12 weeks in subjects with COPD. www.gsk‐clinicalstudyregister.com/files2/gsk‐114930‐clinical‐study‐report‐redact... (first received 26 March 2013).
Donohue 2015b {published and unpublished data}
-
- Donohue JF, Worsley S, Zhu CQ, Hardaker L, Church A. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate‐to‐severe COPD and infrequent exacerbations. Respiratory Medicine 2015;109(7):870‐81. [PUBMED: 26006754 ] - PubMed
-
- GSK DB2114951. DB2114951: a randomized, multi‐center, double‐blind, double‐dummy, parallel group study to evaluate the efficacy umeclidinium/vilanterol compared with fluticasone propionate/salmeterol over 12 weeks in subjects with COPD. www.gsk‐clinicalstudyregister.com/files2/gsk‐114951‐protocol‐redact.pdf (first received 29 July 2013).
Donohue 2016a {published and unpublished data}
-
- Donohue JF, Soong W, Wu X, Shrestha P, Lei A. Long‐term safety of aclidinium bromide/formoterol fumarate fixed‐dose combination: results of a randomized 1‐year trial in patients with COPD. Respiratory Medicine 2016;116:41‐8. [PUBMED: 27296819 ] - PubMed
-
- The Federal Joint Committee (G‐BA) in Germany. Resolution by the Federal Joint Committee on an amendment to the Pharmaceutical Directive (AM‐RL): Appendix XII – Resolutions on the benefit assessment of pharmaceuticals with new active ingredients, in accordance with the German Social Code, Book Five (SGB V), section 35a aclidinium bromide/formoterol. www.english.g‐ba.de/downloads/91‐1028‐156/Aclidinium%20bromide_formoterol_en.pdf (accessed prior to 15 August 2018).
Dransfield 2014 {published and unpublished data}
-
- Dransfield MT, Feldman G, Korenblat P, LaForce CF, Locantore N, Pistolesi M, et al. Efficacy and safety of once‐daily fluticasone furoate/vilanterol (100/25 mcg) versus twice‐daily fluticasone propionate/salmeterol (250/50 mcg) in COPD patients. Respiratory Medicine 2014;108(8):1171‐9. [PUBMED: 24998880 ] - PubMed
-
- GSK HZC112352. A 12‐week study to evaluate the 24‐hour pulmonary function profile of fluticasone furoate/vilanterol (FF/VI) inhalation powder 100/25 mcg once daily compared with fluticasone propionate/salmeterol inhalation powder 250/50 mcg twice daily in subjects with chronic obstructive pulmonary disease (COPD). www.gsk‐clinicalstudyregister.com/files2/gsk‐112352‐clinical‐study‐report‐redact... (first received 18 March 2011).
-
- GSK HZC113109. A 12‐week study to evaluate the 24‐hour pulmonary function profile of fluticasone furoate/vilanterol (FF/VI) inhalation powder 100/25 mcg once daily compared with fluticasone propionate/salmeterol inhalation powder 250/50 mcg twice daily in subjects with chronic obstructive pulmonary disease (COPD). www.gsk‐clinicalstudyregister.com/files2/gsk‐113109‐clinical‐study‐report‐redact... (first received 18 March 2011).
-
- GSK RLV116974. A 12‐week study to evaluate the 24‐hour pulmonary function profile of fluticasone furoate/vilanterol (FF/VI) inhalation powder 100/25 mcg once daily compared with fluticasone propionate/salmeterol inhalation powder 250/50 mcg twice daily in subjects with chronic obstructive pulmonary disease (COPD). www.gsk‐clinicalstudyregister.com/files2/gsk‐116974‐clinical‐study‐report‐redact... (first received 15 October 2012).
Feldman 2016 {published and unpublished data}
-
- Feldman G, Maltais F, Khindri S, Vahdati‐Bolouri M, Church A, Fahy WA, et al. A randomized, blinded study to evaluate the efficacy and safety of umeclidinium 62.5 μg compared with tiotropium 18 μg in patients with COPD. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:719‐30. [PUBMED: 27103795 ] - PMC - PubMed
-
- GSK573719. A randomized, blinded, double‐dummy, parallel‐group study to evaluate the efficacy and safety of umeclidinium (UMEC) 62.5 mcg compared with tiotropium 18 mcg in subjects with chronic obstructive pulmonary disease (COPD). www.gsk‐clinicalstudyregister.com/files2/gsk‐201316‐clinical‐study‐report‐redact... (first received 30 September 2014).
Ferguson 2008 {published and unpublished data}
-
- Ferguson GT, Anzueto A, Fei R, Emmett A, Knobil K, Kalberg C. Effect of fluticasone propionate/salmeterol (250/50 microg) or salmeterol (50 microg) on COPD exacerbations. Respiratory Medicine 2008;102(8):1099‐108. [PUBMED: 18614347] - PubMed
-
- GSK SCO40043. A randomized, double‐blind, parallel group, 52‐week study to compare the effect of fluticasone propionate/salmeterol Diskus inhaler combination product 250/50mcg twice daily with salmeterol Diskus inhaler 50mcg twice daily on the annual rate of moderate/severe exacerbations in subjects with chronic obstructive pulmonary disease (COPD). www.gsk‐clinicalstudyregister.com/files2/gsk‐sco40043‐clinical‐study‐report‐reda... (first received 26 October 2004).
Ferguson 2016 {published and unpublished data}
-
- Ferguson GT, Taylor AF, Thach C, Wang Q, Schubert‐Tennigkeit AA, Patalano F, et al. Long‐term maintenance bronchodilation with indacaterol/glycopyrrolate versus indacaterol in moderate‐to‐severe COPD patients: the FLIGHT 3 study. International Journal of Chronic Obstructive Pulmonary Disease 2016;3(4):716‐28. [PUBMED: 28848898] - PMC - PubMed
Ferguson 2017 {published and unpublished data}
-
- Ferguson GT, Tashkin DP, Skärby T, Jorup C, Sandin K, Greenwood M, et al. Effect of budesonide/formoterol pressurized metered‐dose inhaler on exacerbations versus formoterol in chronic obstructive pulmonary disease: the 6‐month, randomized RISE (Revealing the Impact of Symbicort in reducing Exacerbations in COPD) study. Respiratory Medicine 2017;132:31‐41. [PUBMED: 29229103] - PubMed
-
- NCT02157935. Comparing the efficacy of Symbicort pMDI and formoterol Turbuhaler in reducing exacerbations in patients with chronic obstructive pulmonary disease (RISE) [A phase IIIB, 6‐month, double‐blind, double‐dummy, randomized, parallel‐group, multicenter exacerbation study of Symbicort pressurized metered‐dose inhaler (pMDI) 160/4.5 μg x 2 actuations twice‐daily compared to formoterol Turbuhaler 4.5 μg x 2 inhalations twice‐daily in chronic obstructive pulmonary disease (COPD) patients]. clinicaltrials.gov/ct2/show/NCT02157935 (first received 6 June 2014).
Fukuchi 2013 {published and unpublished data}
-
- Fukuchi Y, Samoro R, Fassakhov R, Taniguchi H, Ekelund J, Carlsson LG, et al. Budesonide/formoterol via Turbuhaler versus formoterol via Turbuhaler in patients with moderate to severe chronic obstructive pulmonary disease: phase III multinational study results. Respirology 2013;18(5):866‐73. [PUBMED: 23551359] - PubMed
-
- NCT 01069289. Efficacy and safety study of Symbicort Turbuhaler versus Oxis Turbuhaler in chronic obstructive pulmonary disease (COPD) patients (SUMIRE) [A phase III, 12‐week, double‐blind, randomised, parallel‐group, active controlled, multinational, efficacy and safety study of Symbicort Turbuhaler 160/4.5 μg 2 inhalations bid compared to Oxis Turbuhaler 4.5 μg 2 inhalations bid in patients with chronic obstructive pulmonary disease (COPD)]. clinicaltrials.gov/ct2/show/study/NCT01069289 (first received 17 February 2010).
GLOW4 2012 {published and unpublished data}
-
- NCT01119937. Long term safety and tolerability of NVA237 versus tiotropium in Japanese patients (GLOW4) [A 52‐week treatment, multi‐center, randomized, open label, parallel group study to assess the long term safety and tolerability of NVA237 (50µg o.d.) using tiotropium (18µg o.d.) as an active control in Japanese patients with moderate to severe chronic obstructive pulmonary disease]. clinicaltrials.gov/ct2/show/NCT01119937 (first received 10 May 2010).
Hagedorn 2013 {published and unpublished data}
-
- Hagedorn C, Kässner F, Banik N, Ntampakas P, Fielder K. Influence of salmeterol/fluticasone via single versus separate inhalers on exacerbations in severe/very severe COPD. Respiratory Medicine 2013;107(4):542‐9. [PUBMED: 23337300 ] - PubMed
Hanania 2003 {published and unpublished data}
-
- GSK FCA3007. A randomized, double‐blind, placebo‐controlled, parallel‐group, trial evaluating the safety and efficacy of the Diskus formulation of salmeterol 50mcg twice daily and fluticasone propionate 250mcg twice daily individually and in combination as compared to placebo in COPD patients. www.gsk‐clinicalstudyregister.com/files2/sfca3007‐clinical‐study‐report‐redact‐v... (first received 10 November 1998).
-
- Hanania NA, Darken P, Horstman D, Reisner C, Lee B, Davis S, et al. The efficacy and safety of fluticasone propionate (250 microg)/salmeterol (50 microg) combined in the Diskus inhaler for the treatment of COPD. Chest 2003;124(3):834‐43. [PUBMED: 12970006] - PubMed
Hanania 2017 {published and unpublished data}
-
- Hanania NA, Tashkin DP, Kerwin EM, Donohue JF, Denenberg M, O'Donnell DE, et al. Long‐term safety and efficacy of glycopyrrolate/formoterol metered dose inhaler using novel Co‐Suspension™ Delivery Technology in patients with chronic obstructive pulmonary disease. Respiratory Medicine 2017;126:105‐15. [PUBMED: 28427541] - PubMed
Hoshino 2013 {published and unpublished data}
-
- Hoshino M, Ohtawa J. Effects of tiotropium and salmeterol/fluticasone propionate on airway wall thickness in chronic obstructive pulmonary disease. Respiration 2013;86(4):280‐7. [PUBMED: 23880883 ] - PubMed
Hoshino 2014 {published and unpublished data}
-
- Hoshino M, Ohtawa J. Computed tomography assessment of airway dimensions with combined tiotropium and indacaterol therapy in COPD patients. Respirology 2014;19(3):403‐10. [PUBMED: 24708031 ] - PubMed
Hoshino 2015 {published and unpublished data}
-
- Hoshino M, Ohtawa J, Akitsu K. Comparison of airway dimensions with once daily tiotropium plus indacaterol versus twice daily Advair in chronic obstructive pulmonary disease. Pulmonary Pharmacology & Therapeutics 2015;30:128‐33. [PUBMED: 25183687 ] - PubMed
Jones 2011 {published and unpublished data}
-
- Jones PW, Mahler DA, Gale R, Owen R, Kramer B. Profiling the effects of indacaterol on dyspnoea and health status in patients with COPD. Respiratory Medicine 2011;105(6):892‐9. [PUBMED: 21397482] - PubMed
Kalberg 2016 {published and unpublished data}
-
- GSK DB2116961. Study DB2116961, a multicentre, randomised, blinded, parallel group study to compare UMEC/VI (umeclidinium/vilanterol) in a fixed dose combination with indacaterol plus tiotropium in symptomatic subjects with moderate to very severe COPD. www.gsk‐clinicalstudyregister.com/files2/gsk‐116961‐clinical‐study‐report‐redact... (first received 15 October 2014).
Kardos 2007 {published and unpublished data}
-
- Kardos P, Wencker M, Glaab T, Vogelmeier C. Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2007;175(2):144‐9. [PUBMED: 17053207] - PubMed
Kerwin 2012a {published and unpublished data}
-
- NCT00929110. A 52‐week treatment, randomized, double‐blind, placebo‐controlled, with open‐label tiotropium, parallel‐group study to assess the efficacy, safety, and tolerability of glycopyrronium bromide (NVA237) in patients with chronic obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT00929110 (first received 26 June 2009).
Kerwin 2017 {published and unpublished data}
-
- GSK DB2116960. A randomized, double‐dummy, parallel group, multicenter trial comparing the efficacy and safety of UMEC/VI (a fixed combination of umeclidinium and vilanterol) with tiotropium in subjects with COPD who continue to have symptoms on tiotropium. www.gsk‐clinicalstudyregister.com/files2/gsk‐116960‐clinical‐study‐report‐redact... (first received 15 September 2014).
-
- Kerwin EM, Kalberg CJ, Galkin DV, Zhu CQ, Church A, Riley JH. Umeclidinium/vilanterol as step‐up therapy from tiotropium in patients with moderate COPD: a randomized, parallel‐group, 12‐week study. International Journal of Chronic Obstructive Pulmonary Disease 2017;12:745‐55. [PUBMED: 28280319] - PMC - PubMed
Koch 2014 {published and unpublished data}
-
- Koch A, Pizzichini E, Hamilton A, Hart L, Korducki L, Salvo MC, et al. Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat versus placebo and formoterol twice daily in patients with GOLD 2‐4 COPD: results from two replicate 48‐week studies. International Journal of Chronic Obstructive Pulmonary Disease 2014;9:697‐714. [PUBMED: 25045258 ] - PMC - PubMed
Kornmann 2011 {published and unpublished data}
-
- Kornmann O, Dahl R, Centanni S, Dogra A, Owen R, Lassen C, et al. Once‐daily indacaterol versus twice‐daily salmeterol for COPD: a placebo‐controlled comparison. European Respiratory Journal 2011;37(2):273‐9. [PUBMED: 20693243] - PubMed
Koser 2010 {published and unpublished data}
-
- GlaxoSmithKline. A randomized, double‐blind, double‐dummy, parallel group 12‐week comparison of the efficacy and safety of fluticasone propionate/salmeterol hydrofluoroalkane 134a metered‐dose‐inhaler 230/42mcg twice‐daily with fluticasone propionate/salmeterol diskus 250/50mcg twice‐daily in subjects with copd. www.gsk‐clinicalstudyregister.com/files2/adc111117‐clinical‐study‐report‐redact.pdf 2014.
-
- Koser A, Westerman J, Sharma S, Emmett A, Crater GD. Safety and efficacy of fluticasone propionate/salmeterol hydrofluoroalkane 134a metered‐dose‐inhaler compared with fluticasone propionate/salmeterol diskus in patients with chronic obstructive pulmonary disease. Open Respiratory Medicine Journal 2010;4:86‐9. [PUBMED: 21253451] - PMC - PubMed
Mahler 2002 {published and unpublished data}
-
- GlaxoSmithKline. A randomized, double‐blind, placebo‐controlled, parallel‐group, trial evaluating the safety and efficacy of the Diskus formulations of salmeterol 50mcg twice daily and fluticasone propionate 500mcg twice daily individually and in combination as compared to placebo in COPD patients. www.gsk‐clinicalstudyregister.com/files2/gsk‐sfca3006‐clinical‐study‐report‐reda... 2015.
-
- Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2002;166(8):1084‐91. [PUBMED: 12379552] - PubMed
Mahler 2012a {published and unpublished data}
-
- Mahler DA, D'Urzo A, Bateman ED, Ozkan SA, White T, Peckitt C, et al. Concurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomised, double‐blind comparison. Thorax 2012;67(9):781‐8. [PUBMED: 22544891 ] - PubMed
-
- Novartis. A randomized, double‐blind, controlled, parallel group, 12‐week treatment study to compare the efficacy and safety of the combination of indacaterol 150 μg once daily with open label tiotropium 18 μg once daily versus open label tiotropium 18 μg once daily in patients with moderate‐to severe chronic obstructive pulmonary disease. www.novctrd.com/CtrdWeb/displaypdf.nov?trialresultid=3901 2011.
Mahler 2012b {published and unpublished data}
-
- Mahler DA, D'Urzo A, Bateman ED, Ozkan SA, White T, Peckitt C, et al. Concurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomised, double‐blind comparison. Thorax 2012;67(9):781‐8. [PUBMED: 22544891 ] - PubMed
-
- Novartis. A randomized, double‐blind, controlled, parallel group, 12‐week treatment study to compare the efficacy and safety of the combination of indacaterol 150 μg once daily with open label tiotropium 18 μg once daily versus open label tiotropium 18 μg once daily in patients with moderate‐to severe chronic obstructive pulmonary disease. www.novctrd.com/CtrdWeb/displaypdf.nov?trialresultid=3903 2011.
Mahler 2015a {published and unpublished data}
-
- Mahler DA, Kerwin E, Ayers T, FowlerTaylor A, Maitra S, Thach C, et al. FLIGHT1 and FLIGHT2: efficacy and safety of QVA149 (indacaterol/glycopyrrolate) versus its monocomponents and placebo in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2015;192(9):1068‐79. [PUBMED: 26177074 ] - PubMed
Mahler 2015b {published and unpublished data}
-
- Mahler DA, Kerwin E, Ayers T, FowlerTaylor A, Maitra S, Thach C, et al. FLIGHT1 and FLIGHT2: efficacy and safety of QVA149 (indacaterol/glycopyrrolate) versus its monocomponents and placebo in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2015;192(9):1068‐79. - PubMed
Mahler 2016 {published and unpublished data}
-
- Mahler DA, Gifford AH, Satti A, Jessop N, Eckert JH, D'Andrea P, et al. Long‐term safety of glycopyrrolate: a randomized study in patients with moderate‐to‐severe COPD (GEM3). Respiratory Medicine 2016;115:39‐45. [PUBMED: 27215502 ] - PubMed
Maleki‐Yazdi 2014 {published and unpublished data}
-
- GlaxoSmithKline. A multicenter, trial comparing the efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg once daily with tiotropium 18 mcg once daily over 24 weeks in subjects with chronic obstructive pulmonary disease (COPD). www.gsk‐clinicalstudyregister.com/files2/gsk‐117115‐clinical‐study‐report‐redact... Dec 03, 2013.
-
- Maleki‐Yazdi MR, Singh D, Anzueto A, Tombs L, Fahy WA, Naya I. Assessing short‐term deterioration in maintenance‐naïve patients with COPD receiving umeclidinium/vilanterol and tiotropium: a pooled analysis of three randomized trials. Advances in Therapy 2017;33(12):2188‐99. [PUBMED: 27796912 ] - PMC - PubMed
Martinez 2017a {published and unpublished data}
-
- Martinez FJ, Rabe KF, Ferguson GT, Fabbri LM, Rennard S, Feldman GJ, et al. Efficacy and safety of glycopyrrolate/formoterol metered dose inhaler formulated using co‐suspension delivery technology in patients with COPD. Chest 2017;151(2):340‐57. [PUBMED: 27916620 ] - PubMed
Martinez 2017b {published and unpublished data}
-
- Martinez FJ, Rabe KF, Ferguson GT, Fabbri LM, Rennard S, Feldman GJ, et al. Efficacy and safety of glycopyrrolate/formoterol metered dose inhaler formulated using co‐suspension delivery technology in patients with COPD. Chest 2017;151(2):340‐57. - PubMed
NCT00876694 2011 {unpublished data only}
-
- NCT00876694. A 52‐week treatment, multi‐center, randomized, open label, parallel group study to assess the long term safety and efficacy of indacaterol (300 µg o.d.) using salmeterol (50 µg b.i.d.) as an active control in Japanese patients with chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT00876694 (first received 7 April 2009).
NCT01536262 2014 {unpublished data only}
-
- NCT01536262. Japan long‐term safety for tiotropium plus olodaterol [A randomised, double‐blind, parallel‐group study to assess the safety and efficacy of 52 weeks of once daily treatment of orally inhaled tiotropium + olodaterol fixed‐dose combination (2.5µg / 5µg, 5µg / 5µg ) and olodaterol (5 µg) delivered by the RESPIMAT inhaler in Japanese patients with chronic obstructive pulmonary disease (COPD)]. clinicaltrials.gov/ct2/show/NCT01536262 (first received 22 February 2012).
Ohar 2014 {published and unpublished data}
-
- GSK ADC113874. A randomized, double‐blind, parallel group, multicenter study of the effects of fluticasone propionate/salmeterol combination product 250/50mcg bid (Advair Diskus™) in comparison to salmeterol 50mcg bid (Serevent Diskus™) on the rate of exacerbations of COPD following hospitalization. www.gsk‐clinicalstudyregister.com/files2/gsk‐113874‐clinical‐study‐report‐redact... (fist received 30 April 2010).
Pepin 2014 {published and unpublished data}
-
- GSK HZC115247. A 12 week study to evaluate the effect of fluticasone furoate (FF, GW685698)/vilanterol (VI, GW642444) 100/25 mcg inhalation powder delivered once daily via a novel dry powder inhaler (NDPI) on arterial stiffness compared with tiotropium bromide 18 mcg delivered once daily via a HandiHaler in subjects with chronic obstructive pulmonary disease (COPD). www.gsk‐clinicalstudyregister.com/files2/gsk‐115247‐clinical‐study‐report‐redact... (first received 23 April 2012).
-
- Pepin JL, Cockcroft JR, Midwinter D, Sharma S, Rubin DB, Andreas S. Long‐acting bronchodilators and arterial stiffness in patients with COPD: a comparison of fluticasone furoate/vilanterol with tiotropium. Chest 2014;146(6):1521‐30. [PUBMED: 25058845 ] - PubMed
Perng 2009 {published and unpublished data}
-
- Perng DW, Tao CW, Su KC, Tsai CC, Liu LY, Lee YC. Anti‐inflammatory effects of salmeterol/fluticasone, tiotropium/fluticasone or tiotropium in COPD. European Respiratory Journal 2009;33(4):778‐84. [PUBMED: 19129278] - PubMed
RADIATE 2016 {unpublished data only}
-
- Larbig M, Taylor AF, Maitra S, Schubert‐Tennigkeit A, Banerji D. Efficacy and safety of IND/GLY (indacaterol/glycopyrronium) versus placebo and tiotropium in symptomatic patients with moderate‐to‐severe COPD: the 52‐week RADIATE study. Respirology 2015;20 (suppl 3):A438.
-
- NCT01610037. Comparison of long‐term safety of the combination product QVA149A against placebo and standard of care treatment in chronic obstructive pulmonary disease patients with moderate to severe airflow limitation [A placebo and active controlled study to assess the long‐term safety of once daily QVA149 for 52 weeks in chronic obstructive pulmonary disease (COPD) patients with moderate to severe airflow limitation]. clinicaltrials.gov/ct2/show/NCT01610037 (first received 1 June 2012).
Rennard 2009 {published and unpublished data}
-
- Rennard SI, Tashkin DP, McElhattan J, Goldman M, Ramachandran S, Martin UJ, et al. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered‐dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1‐year randomized controlled clinical trial. Drugs 2009;69(5):549‐65. [PUBMED: 19368417] - PMC - PubMed
Rheault 2016 {published and unpublished data}
-
- GSK 201315. A randomized, parallel‐group, open‐label study to evaluate the efficacy and safety of umeclidinium (UMEC) 62.5 mcg compared with glycopyrronium 44 mcg in subjects with chronic obstructive pulmonary disease (COPD). www.gsk‐clinicalstudyregister.com/files2/gsk‐201315‐clinical‐study‐report‐redact... (first received 26 September 2014).
Rossi 2014 {published and unpublished data}
-
- Rossi A, Molen T, Olmo R, Papi A, Wehbe L, Quinn M, et al. INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. European Respiratory Journal 2014;44(6):1548‐56. [PUBMED: 25359348] - PubMed
Sarac 2016 {published and unpublished data}
-
- Sarac P, Sayıner A. Compare the efficacy and safety of long‐acting anticholinergic and a combination of inhaled steroids and long‐acting beta‐2 agonist in moderate chronic obstructive pulmonary disease. Tuberk Toraks 2016;64(2):112‐8. [PUBMED: 27481077] - PubMed
SCO100470 2006 {unpublished data only}
-
- GSK SCO100470. A multicentre, randomised, double‐blind, parallel group, 24‐week study to compare the effect of the salmeterol/fluticasone propionate combination product 50/250mcg, with salmeterol 50mcg both delivered twice daily via the Diskus/Accuhaler inhaler on lung function and dyspnoea in subjects with chronic obstructive pulmonary disease (COPD). www.gsk‐clinicalstudyregister.com/files2/gsk‐sco100470‐clinical‐study‐report‐red... (first received 22 June 2004).
SCO40034 2005 {unpublished data only}
-
- GSK SCO40034. A multicentre, randomised, double‐blind, double dummy, parallel group 12‐week exploratory study to compare the effect of the salmeterol/fluticasone propionate combination product (Seretide™) 50/500mcg bd via the Diskus™/Accuhaler™ inhaler with tiotropium bromide 18 mcg od via the HandiHaler inhalation device on efficacy and safety in patients with chronic obstructive pulmonary disease (COPD). www.gsk‐clinicalstudyregister.com/files2/23678.pdf (first received 3 March 2003).
SCO40041 2008 {unpublished data only}
-
- GSK SCO40041. A randomized, double‐blind, parallel‐group clinical trial evaluating the effect of the fluticasone propionate/salmeterol combination product 250/50mcg twice daily via Diskus inhaler versus salmeterol 50mcg twice daily via Diskus inhaler on bone mineral density in subjects with chronic obstructive pulmonary disease (COPD). www.gsk‐clinicalstudyregister.com/files2/gsk‐sco40041‐clinical‐study‐report‐reda... (first received 28 April 2004).
Sharafkhaneh 2012 {published and unpublished data}
-
- Sharafkhaneh A, Southard JG, Goldman M, Uryniak T, Martin UJ. Effect of budesonide/formoterol pMDI on COPD exacerbations: a double‐blind, randomized study. Respiratory Medicine 2012;106(2):257‐68. [PUBMED: 22033040 ] - PubMed
Singh 2014 {published and unpublished data}
-
- European Medicines Agency. Assessment report Duaklir Genuair. www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_r... (accessed prior to 29 August 2018).
-
- Singh D, Jones PW, Bateman ED, Korn S, Serra C, Molins E, et al. Efficacy and safety of aclidinium bromide/formoterol fumarate fixed‐dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM‐COPD): a multicentre, randomised study. BMC Pulmonary Medicine 2014;14:178. [PUBMED: 25404569] - PMC - PubMed
-
- The Federal Joint Committee (G‐BA), Germany. Resolution by the Federal Joint Committee on an amendment to the Pharmaceutical Directive (AM‐RL): Appendix XII – Resolutions on the benefit assessment of pharmaceuticals with new active ingredients, in accordance with the German Social Code, Book Five (SGB V), section 35a aclidinium bromide/formoterol. www.english.g‐ba.de/downloads/91‐1028‐156/Aclidinium%20bromide_formoterol_en.pdf (accessed prior to 29 August 2018).
Singh 2015a {published and unpublished data}
-
- Singh D, Ferguson GT, Bolitschek J, Grönke L, Hallmann C, Bennett N, et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respiratory Medicine 2015;109(10):1312‐9. - PubMed
Singh 2015a&b {published and unpublished data}
-
- Singh D, Ferguson GT, Bolitschek J, Grönke L, Hallmann C, Bennett N, et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respiratory Medicine 2015;109(10):1312‐9. [PUBMED: 26320402] - PubMed
Singh 2015b {published and unpublished data}
-
- Singh D, Ferguson GT, Bolitschek J, Grönke L, Hallmann C, Bennett N, et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respiratory Medicine 2015;109(10):1312‐9. - PubMed
Singh 2015c {published and unpublished data}
-
- GSK DB2116134. DB2116134: a randomized, multi‐center, double‐blind, double‐dummy, parallel group study to evaluate the efficacy and safety of umeclidinium bromide/vilanterol compared with fluticasone propionate/salmeterol over 12 weeks in subjects with COPD. www.gsk‐clinicalstudyregister.com/files2/gsk‐116134‐clinical‐study‐report‐redact... (first received 2 April 2013).
Szafranski 2003 {published and unpublished data}
-
- Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. European Respiratory Journal 2003;21(1):74‐81. [PUBMED: 12570112] - PubMed
Tashkin 2008 {published and unpublished data}
-
- Tashkin DP, Rennard SI, Martin P, Ramachandran S, Martin UJ, Silkoff PE, et al. Efficacy and safety of budesonide and formoterol in one pressurized metered‐dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease: results of a 6‐month randomized clinical trial. Drugs 2008;68(14):1975‐2000. [PUBMED: 18778120] - PubMed
Tashkin 2009 {published and unpublished data}
-
- Tashkin DP, Pearle J, Lezzoni D, Varghese ST. Formoterol and tiotropium compared with tiotropium alone for treatment of COPD. COPD 2009;6(1):17‐25. [PUBMED: 19229704] - PubMed
Tashkin 2012a {published and unpublished data}
-
- Tashkin DP, Doherty DE, Kerwin E, Matiz‐Bueno CE, Knorr B, Shekar T, et al. Efficacy and safety characteristics of mometasone furoate/formoterol fumarate fixed‐dose combination in subjects with moderate to very severe COPD: findings from pooled analysis of two randomized, 52‐week placebo‐controlled trials. International Journal of Chronic Obstructive Pulmonary Disease 2012;7:73‐86. - PMC - PubMed
Tashkin 2012a&b {published and unpublished data}
-
- Doherty DE, Tashkin DP, Kerwin E, Knorr BA, Shekar T, Banerjee S, et al. Effects of mometasone furoate/formoterol fumarate fixed‐dose combination formulation on chronic obstructive pulmonary disease (COPD): results from a 52‐week phase III trial in subjects with moderate‐to‐very severe COPD. International Journal of Chronic Obstructive Pulmonary Disease 2012;7:57‐71. [PUBMED: 22334769 ] - PMC - PubMed
-
- Tashkin DP, Doherty DE, Kerwin E, Matiz‐Bueno CE, Knorr B, Shekar T, et al. Efficacy and safety characteristics of mometasone furoate/formoterol fumarate fixed‐dose combination in subjects with moderate to very severe COPD: findings from pooled analysis of two randomized, 52‐week placebo‐controlled trials. International Journal of Chronic Obstructive Pulmonary Disease 2012;7:73‐86. [PUBMED: 22334770 ] - PMC - PubMed
Tashkin 2012b {published and unpublished data}
-
- Tashkin DP, Doherty DE, Kerwin E, Matiz‐Bueno CE, Knorr B, Shekar T, et al. Efficacy and safety characteristics of mometasone furoate/formoterol fumarate fixed‐dose combination in subjects with moderate to very severe COPD: findings from pooled analysis of two randomized, 52‐week placebo‐controlled trials. International Journal of Chronic Obstructive Pulmonary Disease 2012;7:73‐86. - PMC - PubMed
To 2012 {published and unpublished data}
-
- To Y, Kinoshita M, Lee SH, Hang LW, Ichinose M, Fukuchi Y, et al. Assessing efficacy of indacaterol in moderate and severe COPD patients: a 12‐week study in an Asian population. Respiratory Medicine 2012;106(12):1715‐21. [PUBMED: 23040786] - PubMed
Troosters 2016 {published and unpublished data}
-
- Troosters T, Bourbeau J, Maltais F, Leidy N, Erzen D, Sousa D, et al. Enhancing exercise tolerance and physical activity in COPD with combined pharmacological and non‐pharmacological interventions: PHYSACTO randomised, placebo‐controlled study design. BMJ Open 2016;6(4):e010106. [PUBMED: 27075841] - PMC - PubMed
Vincken 2014 {published and unpublished data}
-
- Vincken W, Aumann J, Chen H, Henley M, McBryan D, Goyal P. Efficacy and safety of coadministration of once‐daily indacaterol and glycopyrronium versus indacaterol alone in COPD patients: the GLOW6 study. International Journal of Chronic Obstructive Pulmonary Disease 2014;9:215‐28. [PUBMED: 24596459 ] - PMC - PubMed
Vogelmeier 2008 {published and unpublished data}
-
- Vogelmeier C, Kardos P, Harari S, Gans SJ, Stenglein S, Thirlwell J. Formoterol mono‐ and combination therapy with tiotropium in patients with COPD: a 6‐month study. Respiratory Medicine 2008;102(11):1511‐20. [PUBMED: 18804362 ] - PubMed
Vogelmeier 2011 {published and unpublished data}
-
- Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten‐van Mölken MP, Beeh KM, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. New England Journal of Medicine 2011;364(12):1093‐103. [PUBMED: 21428765] - PubMed
Vogelmeier 2013a {published and unpublished data}
-
- Vogelmeier CF, Bateman ED, Pallante J, Alagappan VK, D'Andrea P, Chen H, et al. Efficacy and safety of once‐daily QVA149 compared with twice‐daily salmeterol‐fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double‐blind, parallel group study. Lancet Respiratory Medicine 2013;1(1):51‐60. [PUBMED: 24321804] - PubMed
Vogelmeier 2016 {published and unpublished data}
-
- Vogelmeier C, Paggiaro PL, Dorca J, Sliwinski P, Mallet M, Kirsten AM, et al. Efficacy and safety of aclidinium/formoterol versus salmeterol/fluticasone: a phase 3 COPD study. European Respiratory Journal 2016;48(4):1030‐39. [PUBMED: 27492833 ] - PubMed
Vogelmeier 2017 {published and unpublished data}
-
- NCT 01985334. Study to evaluate the efficacy and safety of glycopyrronium or indacaterol maleate and glycopyrronium bromide fixed‐dose combination regarding symptoms and health status in patients with moderate COPD switching from treatment with any standard COPD regimen [A prospective, multicenter, 12‐week, randomized open‐label study to evaluate the efficacy and safety of glycopyrronium (50 micrograms o.d.) or indacaterol maleate and glycopyrronium bromide fixed‐dose combination (110/50 micrograms o.d.) regarding symptoms and health status in patients with moderate chronic obstructive pulmonary disease (COPD) switching from treatment with any standard COPD regimen]. clinicaltrials.gov/ct2/show/NCT01985334 (first received 15 November 2013).
Wedzicha 2008 {published and unpublished data}
-
- GlaxoSmithKline SCO40036. Multicentre, randomised, double‐blind, double dummy, parallel group, 104‐week study to compare the effect of the salmeterol/fluticasone propionate combination product (Seretide*) 50/500mcg delivered twice daily via the Diskus*/Acchuhaler* inhaler with tiotropium bromide 18 mcg delivered once daily via the HandiHaler inhalation device on the rate of health care utilisation exacerbations in subjects with severe chronic obstructive pulmonary disease (COPD). www.gsk‐clinicalstudyregister.com/files2/gsk‐sco40036‐clinical‐study‐report‐reda... (first received 5 June 2003).
-
- Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. American Journal of Respiratory and Critical Care Medicine 2008;177(1):19‐26. [PUBMED: 17916806] - PubMed
Wedzicha 2013 {published and unpublished data}
-
- EudraCT 2009‐013256‐69. A 64‐week treatment, multi‐center, randomized, double‐blind, parallel‐group, active controlled study to evaluate the effect of QVA149 (110/50 μg o.d.) vs NVA237 (50 μg o.d.) and open‐label tiotropium (18 μg o.d.) on COPD exacerbations in patients with severe to very severe chronic obstructive pulmonary disease (COPD). www.clinicaltrialsregister.eu/ctr‐search/trial/2009‐013256‐69/AT#B (first received 28 April 2010).
-
- Wedzicha JA, Decramer M, Ficker JH, Niewoehner DE, Sandström T, Taylor AF, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double‐blind, parallel‐group study. Lancet Respiratory Medicine 2013;1(3):199‐209. [PUBMED: 24429126 ] - PubMed
Wedzicha 2014 {published and unpublished data}
-
- Wedzicha JA, Singh D, Vestbo J, Paggiaro PL, Jones PW, Bonnet‐Gonod F, et al. Extrafine beclomethasone/formoterol in severe COPD patients with history of exacerbations. Respiratory Medicine 2014;108(8):1153‐62. [PUBMED: 24953015 ] - PubMed
Wedzicha 2016 {published and unpublished data}
-
- Wedzicha JA, Banerji D, Chapman KR, Vestbo J, Roche N, Ayers RT, et al. Indacaterol‐glycopyrronium versus salmeterol‐fluticasone for COPD. New England Journal of Medicine 2016;374(23):2222‐34. [PUBMED: 27181606 ] - PubMed
Wise 2013 {published and unpublished data}
-
- Wise RA, Anzueto A, Cotton D, Dahl R, Devins T, Disse B, et al. Tiotropium Respimat inhaler and the risk of death in COPD. New Egyptian Journal of Medicine 2013;369(16):1491‐501. [PUBMED: 23992515 ] - PubMed
Yao 2014 {published and unpublished data}
-
- Yao W, Wang C, Zhong N, Han X, Wu C, Yan X, et al. Effect of once‐daily indacaterol in a predominantly Chinese population with chronic obstructive pulmonary disease: a 26‐week Asia‐Pacific study. Respirology 2014;19(2):231‐8. [PUBMED: 24383720 ] - PubMed
Zhong 2015 {published and unpublished data}
ZuWallack 2014a {published and unpublished data}
-
- ZuWallack R, Allen L, Hernandez G, Ting N, Abrahams R. Efficacy and safety of combining olodaterol Respimat and tiotropium HandiHaler in patients with COPD: results of two randomized, double‐blind, active‐controlled studies. International Journal of Chronic Obstructive Pulmonary Disease 2014;9:1133‐44. - PMC - PubMed
ZuWallack 2014a&b {published and unpublished data}
-
- ZuWallack R, Allen L, Hernandez G, Ting N, Abrahams R. Efficacy and safety of combining olodaterol Respimat and tiotropium HandiHaler in patients with COPD: results of two randomized, double‐blind, active‐controlled studies. International Journal of Chronic Obstructive Pulmonary Disease 2014;9:1133‐44. [PUBMED: 25342898 ] - PMC - PubMed
ZuWallack 2014b {published and unpublished data}
-
- ZuWallack R, Allen L, Hernandez G, Ting N, Abrahams R. Efficacy and safety of combining olodaterol Respimat and tiotropium HandiHaler in patients with COPD: results of two randomized, double‐blind, active‐controlled studies. International Journal of Chronic Obstructive Pulmonary Disease 2014;9:1133‐44. - PMC - PubMed
References to studies excluded from this review
1237.20 {unpublished data only}
-
- NCT01559116. Characterization of 24‐hour lung function profiles of inhaled tiotropium + olodaterol fixed dose combination in patients suffering from chronic obstructive pulmonary disease [Randomised, double‐blind, placebo‐controlled, 6 treatment, 4 period, incomplete cross‐over trial to characterise the 24‐hour lung function profiles of tiotropium + olodaterol fixed dose combination (2.5/5 µg, 5/5 µg), tiotropium (2.5 µg, 5 µg) and olodaterol (5 µg) (oral inhalation, delivered by the Respimat Inhaler) after 6 weeks once daily treatment in patients with chronic obstructive pulmonary disease (COPD) [VIVACITOTM]]. clinicaltrials.gov/ct2/show/NCT01559116 (first received 21 March 2012). [NCT01559116]
1237.4 {unpublished data only}
-
- NCT00696020. Combination of orally inhaled bi1744cl/tiotropium bromide in patients with chronic obstructive pulmonary disease (COPD). Randomised, double‐blind, parallel group study to assess the efficacy and safety of 4 weeks of once daily treatment of 3 doses of orally inhaled bi 1744 cl, each in fixed dose combination with 5 microgram tiotropium bromide (delivered by the Respimat inhaler) compared with 5 microgram tiotropium bromide monoproduct (delivered by the Respimat inhaler) in patients with COPD. clinicaltrials.gov/ct2/show/NCT00696020 (first received June 12, 2008). [NCT00696020]
1237.7 {unpublished data only}
-
- Boehringer Ingelheim. A randomised, placebo‐controlled, double‐blind, single dose, cross‐over study to evaluate the efficacy and safety of orally inhaled tiotropium + olodaterol as both a fixed dose combination and a free combination (both delivered by the Respimat inhaler) in patients with chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT02030535 (first received January 8, 2014). [NCT02030535]
Bateman 2010 {published data only}
Beeh 2014 {published data only}
-
- Beeh KM, Korn S, Beier J, Jadayel D, Henley M, D'Andrea P, et al. Effect of QVA149 on lung volumes and exercise tolerance in COPD patients: the BRIGHT study. Respiratory Medicine 2014 Apr;108(4):584‐92. [PUBMED: 24534204] - PubMed
Beeh 2016 {published data only}
-
- Beeh KM, Derom E, Echave‐Sustaeta J, Grönke L, Hamilton A, Zhai D, et al. The lung function profile of once‐daily tiotropium and olodaterol via Respimat is superior to that of twice‐daily salmeterol and fluticasone propionate via Accuhaler (ENERGITO study). International Journal of Chronic Obstructive Pulmonary Disease 2016;11:193‐205. [PUBMED: 26893551 ] - PMC - PubMed
Berton 2016 {published data only}
Celli 2014 {published data only}
-
- Celli B, Crater G, Kilbride S, Mehta R, Tabberer M, Kalberg CJ, et al. Once‐daily umeclidinium/vilanterol 125/25 mcg in COPD: a randomized, controlled study. Chest 2014;145(5):981‐91. [PUBMED: 24385182 ] - PubMed
CQAB149BIL01 {unpublished data only}
-
- Novartis Pharmaceuticals. A 12 week, multi‐center, randomized, open label study, evaluating the efficacy and safety of treatment regimens that include Onbrez (indacaterol) in patients with moderate to severe COPD (MOVE‐ON Study). clinicaltrials.gov/ct2/show/NCT01232894 (first received November 2, 2010).
CQMF149F2202 {unpublished data only}
-
- Novartis Pharmaceuticals. A randomized, double‐blind, 12‐week treatment, parallel‐group study to evaluate the efficacy and safety of QMF149 (150 µg/160 µg o.d.) compared with salmeterol xinafoate/fluticasone propionate (50 µg/500 µg b.i.d.) in patients with chronic obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT01636076 (first received July 10, 2012).
D'Urzo 2013 {published data only}
-
- D'Urzo A, Kerwin E, Rennard S, He T, Gil EG, Caracta C. One‐year extension study of ACCORD COPD I: safety and efficacy of two doses of twice‐daily aclidinium bromide in patients with COPD. COPD 2013;10(4):500‐10. [PUBMED: 23679347] - PubMed
Dahl 2013 {published data only}
-
- Dahl R, Jadayel D, Alagappan VK, Chen H, Banerji D. Efficacy and safety of QVA149 compared to the concurrent administration of its monocomponents indacaterol and glycopyrronium: the BEACON study. International Journal of Chronic Obstructive Pulmonary Disease 2013;8:501‐8. [PUBMED: 24159259] - PMC - PubMed
Donohue 2014 {published data only}
-
- Donohue JF, Niewoehner D, Brooks J, O'Dell D, Church A. Safety and tolerability of once‐daily umeclidinium/vilanterol 125/25 mcg and umeclidinium 125 mcg in patients with chronic obstructive pulmonary disease: results from a 52‐week, randomized, double‐blind, placebo‐controlled study. Respiratory Research 2014 Jul 11;15:78. [PUBMED: 25015176] - PMC - PubMed
Donohue 2016b {published and unpublished data}
-
- Donohue JF, Singh D, Munzu C, Kilbride S, Church A. Magnitude of umeclidinium/vilanterol lung function effect depends on monotherapy responses: results from two randomised controlled trials. Respiratory Medicine 2016;112:65‐74. [PUBMED: 26797016] - PubMed
Dransfield 2013 {published and unpublished data}
-
- Dransfield MT, Bourbeau J, Jones PW, Hanania NA, Mahler DA, Vestbo J, et al. Once‐daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double‐blind, parallel‐group, randomised controlled trials. Lancet Respiratory Medicine 2013;1(3):210‐23. [PUBMED: 24429127] - PubMed
Fang 2008 {published and unpublished data}
-
- Fang LZ, Liang X, Zhang JQ, Liu L, Fu WP, Zhao ZH, et al. Combination of inhaled salmeterol/fluticasone and tiotropium in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Zhonghua Jie He He Hu Xi Za Zhi 2008;31(11):811‐4. [PUBMED: 19080533] - PubMed
Ferguson 2014 {published data only}
-
- Ferguson GT, Feldman GJ, Hofbauer P, Hamilton A, Allen L, Korducki L, et al. Efficacy and safety of olodaterol once daily delivered via Respimat in patients with GOLD 2–4 COPD: results from two replicate 48‐week studies. International Journal of Chronic Obstructive Pulmonary Disease 2014;9:629‐45. [PUBMED: 24966672] - PMC - PubMed
Gelb 2013 {published data only}
-
- Gelb AF, Tashkin DP, Make BJ, Zhong X, Garcia Gil E, Caracta C, et al. Long‐term safety and efficacy of twice‐daily aclidinium bromide in patients with COPD. Respiratory Medicine 2013;107(12):1957‐65. [PUBMED: 23916502] - PubMed
HZC113108 {unpublished data only}
-
- GlaxoSmithKline. A 24‐week study to evaluate the effect of fluticasone furoate/vilanterol 100/25 mcg inhalation powder delivered once‐daily via a novel dry powder inhaler on arterial stiffness compared with placebo and vilanterol in subjects with chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT01336608 (first received April 18, 2011).
Jones 1997 {published data only}
-
- Jones PW, Bosh TK. Quality of life changes in COPD patients treated with salmeterol. American Journal of Respiratory and Critical Care Medicine 1997;155(4):1283‐9. [PUBMED: 9105068] - PubMed
Jones 2012 {published data only}
-
- Jones PW, Leidy NK, Hareendran A, Lamarca R, Chuecos F, Garcia Gil E. The effect of aclidinium bromide on daily respiratory symptoms of COPD, measured using the Evaluating Respiratory Symptoms in COPD (E‐RS: COPD) diary: pooled analysis of two 6‐month phase III studies. Respiratory Research 2016;17(1):61. [PUBMED: 27215749] - PMC - PubMed
Kerwin 2012b {published data only}
-
- Kerwin EM, D'Urzo AD, Gelb AF, Lakkis H, Garcia Gil E, Caracta CF. Efficacy and safety of a 12‐week treatment with twice‐daily aclidinium bromide in COPD patients (ACCORD COPD I). COPD 2012;9(2):90‐101. [PUBMED: 22320148] - PubMed
Kerwin 2013 {published and unpublished data}
-
- Kerwin EM, Scott‐Wilson C, Sanford L, Rennard S, Agusti A, Barnes N, et al. A randomised trial of fluticasone furoate/vilanterol (50/25 μg; 100/25 μg) on lung function in COPD. Respiratory Medicine 2013;107(4):560‐9. [PUBMED: 23352226] - PubMed
Kurashima 2009 {published data only}
-
- Kurashima K, Hara K, Yoneda K, Kanauchi T, Kagiyama N, Tokunaga D, et al. Changes in lung function and health status in patients with COPD treated with tiotropium or salmeterol plus fluticasone. Respirology 2009;14(2):239‐44. [PUBMED: 19210650] - PubMed
Lipson 2018 {published and unpublished data}
-
- Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, et al. Once‐daily single‐inhaler triple versus dual therapy in patients with COPD. New England Journal of Medicine 2018;378(18):1671‐80. [PUBMED: 29668352] - PubMed
-
- Pascoe SJ, Lipson DA, Locantore N, Barnacle H, Brealey N, Mohindra R, et al. A phase III randomised controlled trial of single‐dose triple therapy in COPD: the IMPACT protocol. European Respiratory Journal 2016;48(2):320‐30. [PUBMED: 27418551] - PubMed
Magnussen 2012 {published data only}
-
- Magnussen H, Paggiaro P, Schmidt H, Kesten S, Metzdorf N, Maltais F. Effect of combination treatment on lung volumes and exercise endurance time in COPD. Respiratory Medicine 2012;106(10):1413‐20. [PUBMED: 22749044] - PubMed
Mahler 2014 {published data only}
-
- Mahler DA, Decramer M, D'Urzo A, Worth H, White T, Alagappan VK, et al. Dual bronchodilation with QVA149 reduces patient‐reported dyspnoea in COPD: the BLAZE study. European Respiratory Journal 2014;43(6):1599‐609. [PUBMED: 24176997] - PubMed
Mahmud 2007 {published data only}
-
- Mahmud AM, Gupta DK, Khan AS, Hassan R, Hossain A, Rahman M, et al. Comparison of once daily tiotropium with twice daily salmeterol in Bangladeshi patients with moderate COPD. Respirology. 2007:12 (Supple 4) A211.
Make 2014 {published data only}
-
- Make BJ, Donohue JF, Soong W, Zhong X, Leselbaum, A, Caracta C. Lung function and safety of aclidinium bromide/formoterol fumarate fixed‐dose combination: results of a 1‐year trial in patients with COPD. American Journal of Respiratory and Critical Care Medicine. 2014; Vol. 189:A6010.
Maltais 2014a {published data only}
-
- GlaxoSmithKline. An Exercise Endurance Study to Evaluate the Effects of Treatment of Chronic Obstructive Pulmonary Disease (COPD) Patients With a Dual Bronchodilator: GSK573719/GW642444. Study A (COPD). clinicaltrials.gov/ct2/show/NCT01328444 (first received April 4, 2011).
Maltais 2014b {published data only}
-
- GlaxoSmithKline. An exercise endurance study to evaluate the effects of treatment of chronic obstructive pulmonary disease (COPD) patients with a dual bronchodilator: GSK573719/GW642444.Study B (COPD). https://clinicaltrials.gov/ct2/show/NCT01323660 (first received March 25, 2011).
Maltais 2018 {published data only}
-
- Maltais F, O'Donnell D, Gáldiz Iturri JB, Kirsten AM, Singh D, Hamilton A, et al. Effect of 12 weeks of once‐daily tiotropium/olodaterol on exercise endurance during constant work‐rate cycling and endurance shuttle walking in chronic obstructive pulmonary disease. Therapeutic Advances in Respiratory Disease 2018;12:1753465818755091. [PUBMED: 29439648] - PMC - PubMed
Martinez 2013 {published and unpublished data}
-
- Martinez FJ, Boscia J, Feldman G, Scott‐Wilson C, Kilbride S, Fabbri L, et al. Fluticasone furoate/vilanterol (100/25; 200/25 μg) improves lung function in COPD: a randomised trial. Respiratory Medicine 2013;107(4):550‐9. [PUBMED: 23332861] - PubMed
MORACTO1 {unpublished data only}
-
- Boehringer Ingelheim. A randomised, double‐blind, 5 treatment arms, 4‐period, incomplete cross‐over study to determine the effect of 6 weeks treatment of orally inhaled tiotropium + olodaterol fixed dose combination (FDC) (2.5 / 5 µg; and 5 / 5 µg) (delivered by the Respimat inhaler) compared with tiotropium (5 µg), olodaterol (5 µg ) and placebo (delivered by the Respimat inhaler) on lung hyperinflation and exercise endurance time during constant work rate cycle ergometry in patients with chronic obstructive pulmonary disease (COPD) [MORACTO TM 1]. clinicaltrials.gov/ct2/show/NCT01533922 (first received February 16, 2012).
MORACTO2 {unpublished data only}
-
- Boehringer Ingelheim. A randomised, double‐blind, 5 treatment arms, 4‐period, incomplete cross‐over study to determine the effect of 6 weeks treatment of orally inhaled tiotropium + olodaterol fixed dose combination (FDC) (2.5 / 5 µg; and 5 / 5 µg) (delivered by the Respimat inhaler) compared with tiotropium (5 µg), olodaterol (5 µg ) and placebo (delivered by the Respimat inhaler) on lung hyperinflation and exercise endurance time during constant work rate cycle ergometry in patients with chronic obstructive pulmonary disease (COPD) [MORACTO TM 2]. clinicaltrials.gov/ct2/show/NCT01533935 (first received February 16, 2012).
PT003016‐00 {unpublished data only}
-
- Pearl Therapeutics. An open‐label, multi‐center, dose indicator study of glycopyrronium and formoterol fumarate (GFF) metered dose inhaler (MDI) in adult subjects with moderate to very severe chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT02268396 (first received October 20, 2014).
Rabe 2008 {published data only}
-
- Rabe KF, Timmer W, Sagkriotis A, Viel K. Comparison of a combination of tiotropium plus formoterol to salmeterol plus fluticasone in moderate COPD. Chest 2008;134(2):255‐62. [PUBMED: 18403672] - PubMed
Rennard 2013 {published data only}
-
- Rennard SI, Scanlon PD, Ferguson GT, Rekeda L, Maurer BT, Garcia Gil E, et al. ACCORD COPD II: a randomized clinical trial to evaluate the 12‐week efficacy and safety of twice‐daily aclidinium bromide in chronic obstructive pulmonary disease patients. Clinical Drug Investigation 2013;33(12):893‐904. [PUBMED: 24085591] - PubMed
Rossi 2012 {published data only}
-
- Rossi A, Centanni S, Cerveri I, Gulotta C, Foresi A, Cazzola M, et al. Acute effects of indacaterol on lung hyperinflation in moderate COPD: a comparison with tiotropium. Respiratory Medicine 2012;106(1):84‐90. [PUBMED: 22035851] - PubMed
SCO100646 {unpublished data only}
-
- GlaxoSmithKline. Clinical evaluation Of GW815SF for chronic obstructive pulmonary disease (chronic bronchitis, emphysema). clinicaltrials.gov/ct2/show/NCT00269126 (first received December 23, 2005).
Siler 2017 {published and unpublished data}
-
- Siler TM, Nagai A, Scott‐Wilson CA, Midwinter DA, Crim C. A randomised, phase III trial of once‐daily fluticasone furoate/vilanterol 100/25 μg versus once‐daily vilanterol 25 μg to evaluate the contribution on lung function of fluticasone furoate in the combination in patients with COPD. Respiratory Medicine 2017;123:8‐17. [PUBMED: 28137501] - PubMed
Singh 2016 {published data only}
-
- Singh D, Schröder‐Babo W, Cohuet G, Muraro A, Bonnet‐Gonod F, Petruzzelli S, et al. The bronchodilator effects of extrafine glycopyrronium added to combination treatment with beclometasone dipropionate plus formoterol in COPD: a randomised crossover study (the TRIDENT study). Respiratory Medicine 2016;114:84‐90. [PUBMED: 27109816] - PubMed
Tashkin 2016 {published data only}
-
- Tashkin DP, Martinez FJ, Rodriguez‐Roisin R, Fogarty C, Gotfried M, Denenberg M, et al. A multicenter, randomized, double‐blind dose‐ranging study of glycopyrrolate/formoterol fumarate fixed‐dose combination metered dose inhaler compared to the monocomponents and open‐label tiotropium dry powder inhaler in patients with moderate‐to‐severe COPD. Respiratory Medicine 2016;120:16‐24. [PUBMED: 27817811] - PubMed
To 2011 {published data only}
-
- To Y, Nishimura M, Fukuchi Y, Kitawaki T, Okino N, et al. Long‐term safety and tolerability of indacaterol versus salmeterol in Japanese COPD patients: a 52‐week open‐labeled study. Respirology. Conference: 16th Congress of the Asian Pacific Society of Respirology. 2011:16:96.
Van Noord 2010 {published data only}
-
- Noord JA, Aumann JL, Janssens E, Smeets JJ, Zaagsma J, Mueller A, et al. Combining tiotropium and salmeterol in COPD: effects on airflow obstruction and symptoms. Respiratory Medicine 2010;104(7):995‐1004. [PUBMED: 20303247] - PubMed
Vestbo 2016 {published and unpublished data}
-
- Vestbo J, Anderson JA, Brook RD, Calverley PM, Celli BR, Crim C, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double‐blind randomised controlled trial. Lancet 2016;30:387. [PUBMED: 27203508] - PubMed
Vogelmeier 2010a {published data only}
-
- Vogelmeier C, Verkindre C, Cheung D, Galdiz JB, Güçlü SZ, Spangenthal S, et al. Safety and tolerability of NVA237, a once‐daily long‐acting muscarinic antagonist, in COPD patients. Pulmonary Pharmacology & Therapeutics 2010;23(5):438‐44. [PUBMED: 28737971] - PubMed
Vogelmeier 2010b {published data only}
Vogelmeier 2013b {published data only}
-
- Vogelmeier C, Fabbri LM, Rabe KF, Beeh KM, Schmidt H, Metzdorf N, et al. Effect of tiotropium vs. salmeterol on exacerbations: GOLD II and maintenance therapy naïve patients. Respiratory Medicine 2013;107(1):75‐83. [PUBMED: 23102611] - PubMed
Watz 2016 {published data only}
-
- Watz H, Mailänder C, Baier M, Kirsten A. Effects of indacaterol/glycopyrronium (QVA149) on lung hyperinflation and physical activity in patients with moderate to severe COPD: a randomised, placebo‐controlled, crossover study (The MOVE Study). BMC Pulmonary Medicine 2016;16(1):95. [PUBMED: 27301417] - PMC - PubMed
Wouters 2005 {published data only}
-
- Wouters EF, Postma DS, Fokkens B, Hop WC, Prins J, Kuipers AF, et al. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomised controlled trial. Thorax 2005;60(6):480‐7. [PUBMED: 15923248] - PMC - PubMed
Zheng 2015 {published data only}
-
- Zheng J, Zhong N, Newlands A, Church A, Goh AH. Efficacy and safety of once‐daily inhaled umeclidinium/vilanterol in Asian patients with COPD: results from a randomized, placebo‐controlled study. International Journal of Chronic Obstructive Pulmonary Disease 2015;10:1753‐67. [PMC4562726] - PMC - PubMed
References to studies awaiting assessment
Calverley 2018 {published data only}
-
- Calverley PM, Anzueto AR, Carter K, Grönke L, Hallmann C, Jenkins C, et al. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): a double‐blind, randomised, parallel‐group, active‐controlled trial. Lancet Respiratory Medicine 2018 May;6(5):337‐44. - PubMed
Papi 2017 {published and unpublished data}
References to ongoing studies
AMPLIFY {unpublished data only}
-
- AstraZeneca. A 24 week treatment, multicenter, randomized, double blinded, double dummy, parallel‐group, clinical trial evaluating the efficacy and safety of aclidinium bromide 400 μg/formoterol fumarate 12 μg fixed‐dose combination bid compared with each monotherapy (aclidinium bromide 400 μg bid and formoterol fumarate 12 μg bid) and tiotropium 18 μg qd when administered to patients with stable chronic obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT02796677 (first received June 13, 2016).
-
- Sethi S, Kerwin EM, Watz H, Ferguson GT, Mroz R, Segarra R, et al. AMPLIFY: A randomized, phase III study evaluating the efficacy and safety of aclidinium/formoterol versus monotherapy in patients with COPD. American Journal of Respiratory and Critical Care Medicine. 2018; Vol. 197:A4241.
AVANT {unpublished data only}
-
- AstraZeneca. A 24‐week treatment, randomised, parallel‐group, double blinded, double‐dummy, multicenter study to assess the efficacy and safety of aclidinium bromide/formoterol fumarate compared with individual components and placebo and aclidinium bromide compared with placebo when administered to patients with stable chronic obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT03022097 (first received January 16, 2017).
FLASH {unpublished data only}
-
- Frith P, Ashmawi S, Krishnamurthy S, Diaz D, Gurgun A, Hours‐Zesiger P, et al. Assessing direct switch to indacaterol/glycopyrronium from salmeterol/fluticasone in moderate to severe symptomatic COPD patients: the FLASH Study. Respirology. 2017:AOL011.
-
- Novartis Pharmaceuticals. Assessment of switching from salmeterol/fluticasone to indacaterol/glycopyrronium in a symptomatic COPD patient cohort (FLASH). clinicaltrials.gov/ct2/show/NCT02516592 (first received August 6, 2015). [PUBMED: NCT02516592]
FLT3510 {unpublished data only}
-
- Mundipharma Research. A randomised double‐blind, double‐dummy parallel group study to compare the efficacy and safety of fluticasone propionate / formoterol fumarate (Flutiform) 500/20 µg bid and 250/10 µg bid versus salmeterol / fluticasone (Seretide) 50/500 µg bid in subjects with chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/ct2/show/NCT02195375 (first received July 21, 2014).
PINNACLE 4 {unpublished data only}
-
- Pearl Therapeutics. A randomized, double‐blind, chronic dosing (24 weeks), placebo‐controlled, parallel group, multi‐center study to assess the efficacy and safety of PT003, PT005, and PT001 in subjects with moderate to very severe COPD, compared with placebo. clinicaltrials.gov/ct2/show/NCT02343458 (first received January 22, 2015).
PT010006 {unpublished data only}
-
- Pearl Therapeutics. A randomized, double‐blind, parallel‐group, 24‐week, chronic‐dosing, multi‐center study to assess the efficacy and safety of PT010, PT003, and PT009 compared with Symbicort Turbuhaler as an active control in subjects with moderate to very severe chronic obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT02497001 (first received July 14, 2015).
Additional references
ATS/ERS 2004
-
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. European Respiratory Journal 2004;23(6):932‐46. - PubMed
Chen 2017
-
- Chen WC, Huang CH, Sheu CC, Chong IW, Chu KA8, Chen YC, et al. Long‐acting beta2‐agonists versus long‐acting muscarinic antagonists in patients with stable COPD: a systematic review and meta‐analysis of randomized controlled trials. Respirology 2017;7:1313‐19. [PUBMED: 28654201] - PubMed
Dechartres 2013
Decramer 2012
Dias 2013a
Dias 2013b
Dias 2013c
Dias 2018
-
- Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ. Network Meta‐Analysis for Decision‐Making. John Wiley & Sons, 2018. [ISBN 1118951727, 9781118951729]
Donohue 2015
-
- Donohue JF, Worsley S, Zhu CQ, Hardaker L, Church A. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate‐to‐severe COPD and infrequent exacerbations. Respiratory Medicine 2015;109(7):870‐81. - PubMed
Egger 1997
Falk 2008
Farne 2015
GOLD 2018
-
- 2018 Global strategy for prevention, diagnosis and management of COPD. goldcopd.org/gold‐reports/ (accessed 31 August 2018).
GRADEpro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 7 July 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2011a
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. - PubMed
Guyatt 2011b
-
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380‐2. - PubMed
Heidari 2012
Higgins 2003
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Horita 2017
-
- Horita N, Goto A, Shibata Y, Ota E, Nakashima K, Nagai K, et al. Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.CD012066.pub2] - DOI - PMC - PubMed
Huisman 2015
Kew 2014
Kume 2014
-
- Kume H, Imbe S, Nishiyama O, Iwanaga T, Higashimoto Y, Tohda Y. Involvement of regulation of KCa channels via Gi, Gs in the synergistic action between anticholinergic agents and β2‐adrenergic receptor agonists in airway smooth muscle. American Journal of Respiratory and Critical Care Medicine 2014;189:A5589.
Mathieu 2009
-
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 2009;302(9):977‐84. - PubMed
McCarthy 2015
Moher 2009
Nannini 2012
Nüesch 2010
Oba 2016a
-
- Oba Y, Sarva ST, Dias S. Efficacy and safety of long‐acting beta‐agonist/long‐acting muscarinic antagonist combinations in COPD: a network meta‐analysis. Thorax 2016;71(1):15‐25. - PubMed
Oba 2016b
-
- Oba Y, Chandran A, Devasahayam J. Long‐acting muscarinic antagonist versus inhaled corticosteroid when added to long‐acting β‐agonist for COPD: a meta‐analysis. COPD 2016;13(6):677‐85. - PubMed
Patel 2014
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rochester 2015
-
- Rochester CL, Vogiatzis I, Holland AE, Lareau SC, Marciniuk DD, Puhan MA, et al. ATS/ERS Task Force on Policy in Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society policy statement. Enhancing implementation, use, and delivery of pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine 2015;192(11):1373‐86. - PubMed
Rodrigo 2017
Schlueter 2016
-
- Schlueter M, Gonzalez‐Rojas N, Baldwin M, Groenke L, Voss F, Reason T. Comparative efficacy of fixed‐dose combinations of long‐acting muscarinic antagonists and long‐acting β2‐agonists: a systematic review and network meta‐analysis. Therapeutic Advances in Respiratory Disease 2016;10(2):89‐104. - PMC - PubMed
Singh 2015d
Spiegelhalter 2002
-
- Spiegelhalter DJ, Best NG, Carlin BP, Linde A. Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society. Series B (Statistical Methodology) 2002;64(4):583‐39.
Suissa 2012
Tricco 2015
Trucchi 2015
Turner 2012
Vogelmeier 2015
-
- Vogelmeier C, Paggiaro PL, Dorca J, Sliwinski P, Mallet M, Kirsten A, et al. Efficacy of aclidinium/formoterol fixed‐dose combination versus salmeterol/fluticasone in COPD. European Respiratory Journal 2015;46(suppl 59):PA2960.
Welsh 2013
WHO 2016
-
- World Health Organization. Chronic obstructive pulmonary disease. Burden of COPD. www.who.int/respiratory/copd/burden/en/ (accessed 17 December 2016).
WinBUGS [Computer program]
-
- Medical Research Council (MRC). WinBUGS. Version 1.4.3. UK: Medical Research Council (MRC), 2007.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

