Non-Graft-versus-Host Disease Ocular Complications after Hematopoietic Cell Transplantation: Expert Review from the Late Effects and Quality of Life Working Committee of the Center for International Blood and Marrow Transplant Research and the Transplant Complications Working Party of the European Society for Blood and Marrow Transplantation
- PMID: 30521975
- PMCID: PMC6511311
- DOI: 10.1016/j.bbmt.2018.11.033
Non-Graft-versus-Host Disease Ocular Complications after Hematopoietic Cell Transplantation: Expert Review from the Late Effects and Quality of Life Working Committee of the Center for International Blood and Marrow Transplant Research and the Transplant Complications Working Party of the European Society for Blood and Marrow Transplantation
Abstract
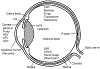
Non-graft-versus-host disease (GVHD) ocular complications are generally uncommon after hematopoietic cell transplantation (HCT) but can cause prolonged morbidity affecting activities of daily living and quality of life. Here we provide an expert review of non-GVHD ocular complications in a collaboration between transplantation physicians and ophthalmologists through the Late Effects and Quality of Life Working Committee of the Center for International Blood and Marrow Transplant Research and the Transplant Complications Working Party of the European Society of Blood and Marrow Transplantation. Complications discussed in this review include cataracts, glaucoma, ocular infections, ocular involvement with malignancy, ischemic microvascular retinopathy, central retinal vein occlusion, retinal hemorrhage, retinal detachment and ocular toxicities associated with medications. We summarize the incidence, risk factors, screening, prevention, and treatment of individual complications and generate evidence-based recommendations. Baseline ocular evaluation before HCT should be considered in all patients who undergo HCT. Follow-up evaluations should be considered according to clinical signs and symptoms and risk factors. Better preventive strategies and treatments remain to be investigated for individual ocular complications after HCT. Both transplantation physicians and ophthalmologists should be knowledgeable about non-GVHD ocular complications and provide comprehensive collaborative team care.
Keywords: Complication; Eye; Hematopoietic cell transplantation; Prevention; Review; Treatment.
Copyright © 2018 American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
References
-
- Socie G, Salooja N, Cohen A, Rovelli A, Carreras E, Locasciulli A, et al. Nonmalignant late effects after allogeneic stem cell transplantation. Blood. 2003;101:3373–85. - PubMed
-
- Couriel D, Carpenter PA, Cutler C, Bolanos-Meade J, Treister NS, Gea-Banacloche J, et al. Ancillary therapy and supportive care of chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2006;12:375–96. - PubMed
-
- Dunn JP, Jabs DA, Wingard J, Enger C, Vogelsang G, Santos G. Bone marrow transplantation and cataract development. Arch Ophthalmol. 1993;111:1367–73. - PubMed