Efficacy and safety of polydioxanone thread embedded at specific acupoints for non-specific chronic neck pain: a study protocol for a randomized, subject-assessor-blinded, sham-controlled pilot trial
- PMID: 30522504
- PMCID: PMC6282385
- DOI: 10.1186/s13063-018-3058-9
Efficacy and safety of polydioxanone thread embedded at specific acupoints for non-specific chronic neck pain: a study protocol for a randomized, subject-assessor-blinded, sham-controlled pilot trial
Abstract
Background: This study aims to evaluate the efficacy and safety of thread-embedding acupuncture (TEA) with polydioxanone thread embedded at various acupoints, compared with sham TEA, for the treatment of non-specific chronic neck pain.
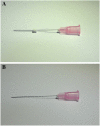
Methods/design: This study will be an 8-week-long, two-armed, parallel, randomized, subject-assessor-blinded, sham-controlled pilot trial. Fifty eligible patients will be randomly allocated into the real TEA group or the sham TEA group. The real TEA group will receive TEA treatment at 14 fixed acupoints in the neck region. The sham TEA group will receive the same treatment as the real TEA group, but with a sham device with the thread removed. Both groups will receive treatment once a week for a total of four sessions. The primary outcome will be the mean change in the visual analog scale (VAS) from baseline to week 6 (2 weeks post intervention). Clinical relevance (ratio of the number of patients with decreases on the VAS of ≥15 mm or with percentiles ≥ 30% and ≥ 50% relative to baseline to the total number of patients), Neck Disability Index, pressure pain threshold, the Hospital Anxiety and Depression Scale, EuroQol 5-Dimensions questionnaire, Patient Global Impression of Change, blinding test, and adverse events will be used to assess secondary outcomes.
Discussion: The results of this study will provide valuable data for a large-scale clinical trial to evaluate the clinical effects of polydioxanone TEA in the treatment of patients with non-specific chronic neck pain.
Trial registration: Clinical Research Information Service (CRIS), Republic of Korea, KCT0002452 . Registered on 6 September 2017.
Keywords: Chronic neck pain; Efficacy; Polydioxanone; Randomized sham-controlled trial; Thread-embedding acupuncture.
Conflict of interest statement
Ethics approval and consent to participate
This trial has been approved by the Institutional Review Board (IRB) of Korean Medicine Hospital of Daejeon University (DJDSKH-17-BM-08; March 2017, protocol version 1.2). This study protocol has been designed according to Korean Good Clinical Practices (GCPs) and the Declaration of Helsinki, and has been registered with the Clinical Research Information Service (CRIS) of the National Research Institution of Health in Korea (identifier: KCT0002452), which is recognized as a registry in the World Health Organization’s network. Other important protocol modifications after protocol publication and publication of trial results will be notified through updates on the trial registration site (CRIS). All documents will be identified by an identification code rather than personal information and will be securely stored in a cabinet with locks in the Clinical Trial Center of DKMHDU. Data processing for statistical analyses will be performed by an independent third party and no interim analyses will be conducted.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Effectiveness and safety of polydioxanone thread embedding acupuncture compared to physical therapy in the treatment of patients with non-specific chronic neck pain: Study protocol for an assessor-blinded, randomized, controlled, clinical trial.Medicine (Baltimore). 2019 Aug;98(32):e16768. doi: 10.1097/MD.0000000000016768. Medicine (Baltimore). 2019. PMID: 31393397 Free PMC article. Clinical Trial.
-
Effectiveness and Safety of Polydioxanone Thread-Embedding Acupuncture as an Adjunctive Therapy for Patients with Chronic Nonspecific Neck Pain: A Randomized Controlled Trial.J Altern Complement Med. 2019 Apr;25(4):417-426. doi: 10.1089/acm.2018.0228. Epub 2018 Dec 5. J Altern Complement Med. 2019. PMID: 30523703 Clinical Trial.
-
Clinical research on the efficacy and safety of thread-embedding acupuncture for treatment of herniated intervertebral disc of the lumbar spine: a protocol for a multicenter, randomized, patient-assessor blinded, controlled, parallel, clinical trial.Trials. 2018 Sep 10;19(1):484. doi: 10.1186/s13063-018-2864-4. Trials. 2018. PMID: 30201029 Free PMC article.
-
Teasing apart quality and validity in systematic reviews: an example from acupuncture trials in chronic neck and back pain.Pain. 2000 May;86(1-2):119-32. doi: 10.1016/s0304-3959(00)00234-7. Pain. 2000. PMID: 10779669
-
Durable Effect of Acupuncture for Chronic Neck Pain: A Systematic Review and Meta-Analysis.Curr Pain Headache Rep. 2024 Sep;28(9):957-969. doi: 10.1007/s11916-024-01267-x. Epub 2024 Jun 10. Curr Pain Headache Rep. 2024. PMID: 38856887 Free PMC article.
Cited by
-
Effectiveness and Safety of Polydioxanone Thread Embedding Acupuncture Compared to Physical Therapy in the Treatment of Patients with Non-Specific Chronic Neck Pain: An Assessor-Blinded, Randomized, Controlled, Clinical Trial.J Pain Res. 2021 Jan 28;14:201-211. doi: 10.2147/JPR.S276941. eCollection 2021. J Pain Res. 2021. PMID: 33536781 Free PMC article.
-
Efficacy and safety of thread embedding acupuncture on knee osteoarthritis: A randomized, controlled, pilot clinical trial.Medicine (Baltimore). 2020 Sep 4;99(36):e21957. doi: 10.1097/MD.0000000000021957. Medicine (Baltimore). 2020. PMID: 32899030 Free PMC article. Clinical Trial.
-
Effectiveness and safety of polydioxanone thread embedding acupuncture compared to physical therapy in the treatment of patients with non-specific chronic neck pain: Study protocol for an assessor-blinded, randomized, controlled, clinical trial.Medicine (Baltimore). 2019 Aug;98(32):e16768. doi: 10.1097/MD.0000000000016768. Medicine (Baltimore). 2019. PMID: 31393397 Free PMC article. Clinical Trial.
References
-
- Hogg-Johnson S, van der Velde G, Carroll LJ, Holm LW, Cassidy JD, Guzman J, et al. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976) 2008;33(4 Suppl):S39–S51. - PubMed
-
- Côté P, Cassidy JD, Carroll LJ, Kristman V. The annual incidence and course of neck pain in the general population: a population-based cohort study. Pain. 2004;112(3):267–273. - PubMed
-
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287. - PubMed
-
- Webb R, Brammah T, Lunt M, Urwin M, Allison T, Symmons D. Prevalence and predictors of intense, chronic, and disabling neck and back pain in the UK general population. Spine. 2003;28(11):1195–1202. - PubMed
-
- Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, et al. Chronic spinal pain and physical-mental comorbidity in the United States: results from the national comorbidity survey replication. Pain. 2005;113(3):331–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical