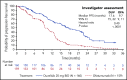
DUO delivers for duvelisib
- PMID: 30523122
- PMCID: PMC6284219
- DOI: 10.1182/blood-2018-10-879650
DUO delivers for duvelisib
Abstract
In this issue of Blood,
Conflict of interest statement
Conflict-of-interest disclosure: J.R.B. has consulted for Verastem Oncology, Gilead Sciences, TG Therapeutics, Janssen, Pharmacyclics, AbbVie, Genentech, Acerta Pharma, AstraZeneca, BeiGene, Loxo Oncology, Sunesis Pharmaceuticals, Sun Pharma, Pfizer, Celgene, Kite Pharma, Redx Pharma, and Astellas Pharma; has received an honorarium from Teva Pharmaceutical Industries and research funding from Verastem Oncology, Gilead Sciences, Sun Pharma, and Loxo Oncology; and has served on Data Safety Monitoring Boards for MorphoSys and Invectys.
Figures
Comment on
-
The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL.Blood. 2018 Dec 6;132(23):2446-2455. doi: 10.1182/blood-2018-05-850461. Epub 2018 Oct 4. Blood. 2018. PMID: 30287523 Free PMC article. Clinical Trial.
References
-
- Dreyling M, Santoro A, Mollica L, et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol. 2017;35(35):3898-3905. - PubMed
-
- Weaver D, Sprott K, Pachter J, et al. Duvelisib inhibition of chemokines in patients with CLL (DUO study) and iNHL (DYNAMO study). J Clin Oncol. 2018;36(suppl 15):12048.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources