GPCR-specific autoantibody signatures are associated with physiological and pathological immune homeostasis
- PMID: 30523250
- PMCID: PMC6283882
- DOI: 10.1038/s41467-018-07598-9
GPCR-specific autoantibody signatures are associated with physiological and pathological immune homeostasis
Abstract
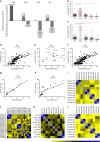
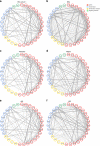
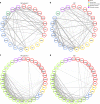
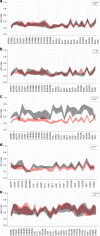
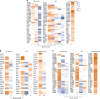
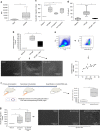
Autoantibodies have been associated with autoimmune diseases. However, studies have identified autoantibodies in healthy donors (HD) who do not develop autoimmune disorders. Here we provide evidence of a network of immunoglobulin G (IgG) autoantibodies targeting G protein-coupled receptors (GPCR) in HD compared to patients with systemic sclerosis, Alzheimer's disease, and ovarian cancer. Sex, age and pathological conditions affect autoantibody correlation and hierarchical clustering signatures, yet many of the correlations are shared across all groups, indicating alterations to homeostasis. Furthermore, we identify relationships between autoantibodies targeting structurally and functionally related molecules, such as vascular, neuronal or chemokine receptors. Finally, autoantibodies targeting the endothelin receptor type A (EDNRA) exhibit chemotactic activity, as demonstrated by neutrophil migration toward HD-IgG in an EDNRA-dependent manner and in the direction of IgG from EDNRA-immunized mice. Our data characterizing the in vivo signatures of anti-GPCR autoantibodies thus suggest that they are a physiological part of the immune system.
Conflict of interest statement
The authors declare that H.H. and K.S.F. are CellTrend managing directors and that Gabriela Riemekasten is an advisor of the company CellTrend and earned an honorarium for her advice between 2011 and 2015. The other authors declare no competing interests.
Figures


References
-
- Ehrlich P. Croonian Lecture: on immunity with special reference to cell life. Proc. R. Soc. Lond. 1899;66:424–448. doi: 10.1098/rspl.1899.0121. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

