The role of dihydrosphingolipids in disease
- PMID: 30523364
- PMCID: PMC11105797
- DOI: 10.1007/s00018-018-2984-8
The role of dihydrosphingolipids in disease
Abstract
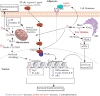
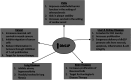
Dihydrosphingolipids refer to sphingolipids early in the biosynthetic pathway that do not contain a C4-trans-double bond in the sphingoid backbone: 3-ketosphinganine (3-ketoSph), dihydrosphingosine (dhSph), dihydrosphingosine-1-phosphate (dhS1P) and dihydroceramide (dhCer). Recent advances in research related to sphingolipid biochemistry have shed light on the importance of sphingolipids in terms of cellular signalling in health and disease. However, dihydrosphingolipids have received less attention and research is lacking especially in terms of their molecular mechanisms of action. This is despite studies implicating them in the pathophysiology of disease, for example dhCer in predicting type 2 diabetes in obese individuals, dhS1P in cardiovascular diseases and dhSph in hepato-renal toxicity. This review gives a comprehensive summary of research in the last 10-15 years on the dihydrosphingolipids, 3-ketoSph, dhSph, dhS1P and dhCer, and their relevant roles in different diseases. It also highlights gaps in research that could be of future interest.
Keywords: 4-HRP fenretinide; Adipocyte; Aging; Airway hypersensitivity; Apoptosis; Autophagy; Cancer; Cardiomyopathy; Ceramide; Ceramide synthase; Diabetes; Dihydroceramide desaturase 1-Des-1; Dihydrosphinganine; FB1 toxicity; Hypoxia; Neurodegenerative; Serine palmitoyl transferase; Sphingosine kinase; Sphingosine-1-phosphate receptors; Sphingosine-1-phosphate—S1P.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures



References
-
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101(7):2070–2075. doi: 10.1073/pnas.0305799101. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

