Phase I/II Study of Stem-Cell Transplantation Using a Single Cord Blood Unit Expanded Ex Vivo With Nicotinamide
- PMID: 30523748
- PMCID: PMC6368416
- DOI: 10.1200/JCO.18.00053
Phase I/II Study of Stem-Cell Transplantation Using a Single Cord Blood Unit Expanded Ex Vivo With Nicotinamide
Abstract
Purpose: Increasing the number of hematopoietic stem and progenitor cells within an umbilical cord blood (UCB) graft shortens the time to hematopoietic recovery after UCB transplantation. In this study, we assessed the safety and efficacy of a UCB graft that was expanded ex vivo in the presence of nicotinamide and transplanted after myeloablative conditioning as a stand-alone hematopoietic stem-cell graft.
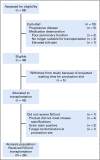
Methods: Thirty-six patients with hematologic malignancies underwent transplantation at 11 sites.
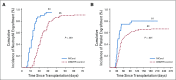
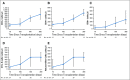
Results: The cumulative incidence of neutrophil engraftment at day 42 was 94%. Two patients experienced secondary graft failure attributable to viral infections. Hematopoietic recovery was compared with that observed in recipients of standard UCB transplantation as reported to the Center for International Blood and Marrow Transplant Research (n = 146). The median time to neutrophil recovery was 11.5 days (95% CI, 9 to 14 days) for recipients of nicotinamide-expanded UCB and 21 days (95% CI, 20 to 23 days) for the comparator ( P < .001). The median time to platelet recovery was 34 days (95% CI, 32 to 42 days) and 46 days (95% CI, 42 to 50 days) for the expanded and the comparator cohorts, respectively ( P < .001). The cumulative incidence of grade 2 to 4 acute graft-versus-host disease (GVHD) at day 100 was 44%, and grade 3 and 4 acute GVHD at day 100 was 11%. The cumulative incidence at 2 years of all chronic GVHD was 40%, and moderate/severe chronic GVHD was 10%. The 2-year cumulative incidences of nonrelapse mortality and relapse were 24% and 33%, respectively. The 2-year probabilities of overall and disease-free survival were 51% and 43%, respectively.
Conclusion: UCB expanded ex vivo with nicotinamide shortens median neutrophil recovery by 9.5 days (95% CI, 7 to 12 days) and median platelet recovery by 12 days (95% CI, 3 to 16.5 days). This trial establishes feasibility, safety, and efficacy of an ex vivo expanded UCB unit as a stand-alone graft.
Trial registration: ClinicalTrials.gov NCT01816230.
Figures


Comment in
-
Cord Blood Expansion: A Clinical Advance.J Clin Oncol. 2019 Feb 10;37(5):363-366. doi: 10.1200/JCO.18.01789. Epub 2018 Dec 21. J Clin Oncol. 2019. PMID: 30576267 No abstract available.
References
-
- Peled T, Shoham H, Aschengrau D, et al: Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol 40:342-355.e1, 2012. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

